Abstract
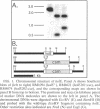
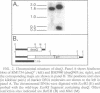
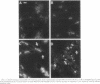
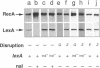
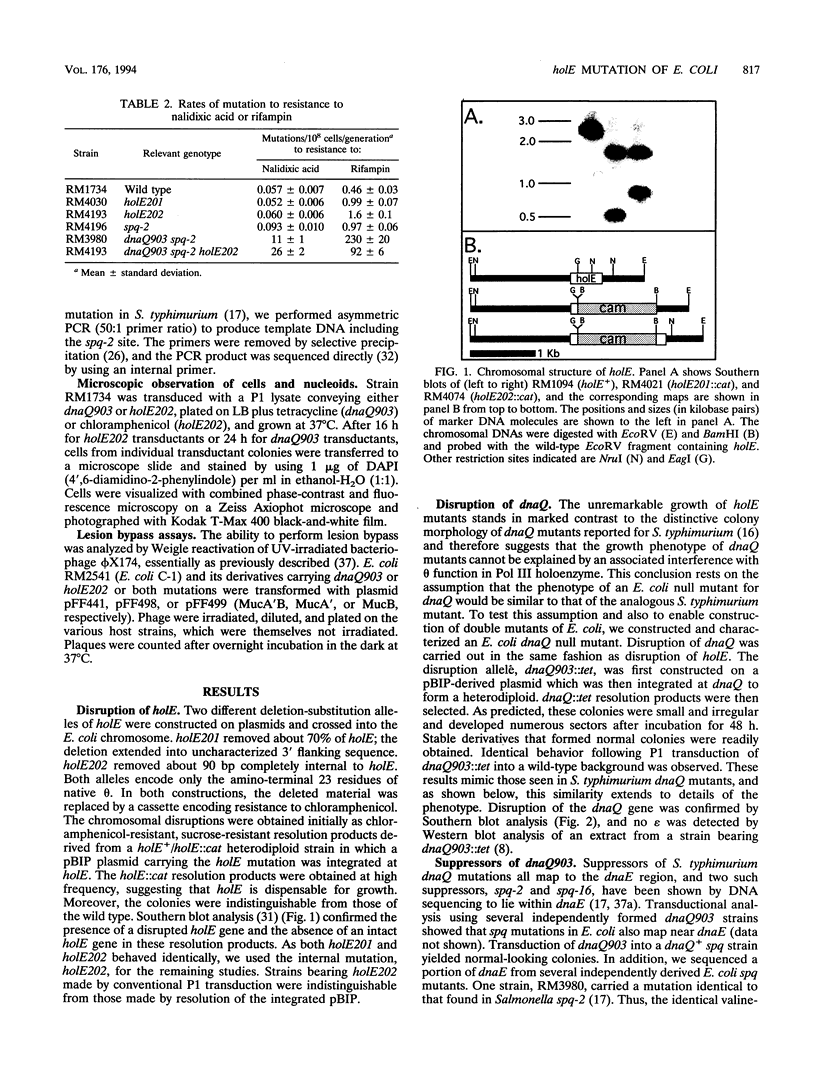
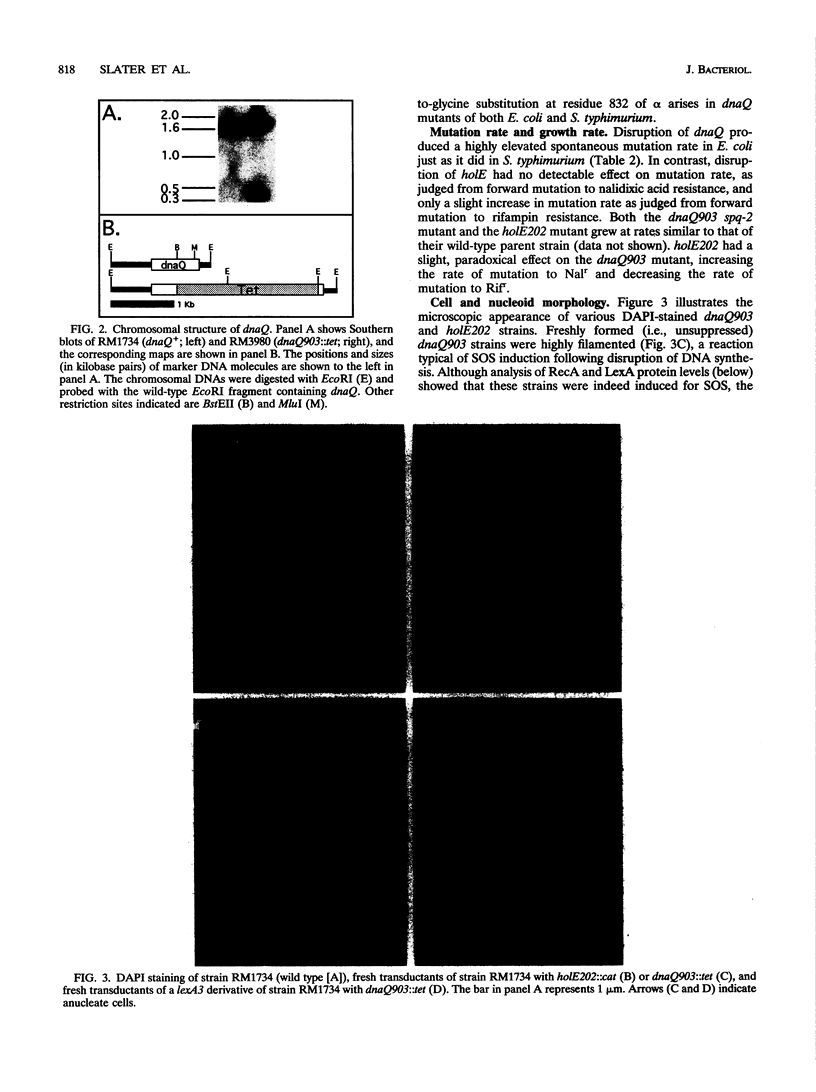
DNA polymerase III holoenzyme is a multiprotein complex responsible for the bulk of chromosomal replication in Escherichia coli and Salmonella typhimurium. The catalytic core of the holoenzyme is an alpha epsilon theta heterotrimer that incorporates both a polymerase subunit (alpha; dnaE) and a proofreading subunit (epsilon; dnaQ). The role of theta is unknown. Here, we describe a null mutation of holE, the gene for theta. A strain carrying this mutation was fully viable and displayed no mutant phenotype. In contrast, a dnaQ null mutant exhibited poor growth, chronic SOS induction, and an elevated spontaneous mutation rate, like dnaQ null mutants of S. typhimurium described previously. The poor growth was suppressible by a mutation affecting alpha which was identical to a suppressor mutation identified in S. typhimurium. A double mutant null for both holE and dnaQ was indistinguishable from the dnaQ single mutant. These results show that the theta subunit is dispensable in both dnaQ+ and mutant dnaQ backgrounds, and that the phenotype of epsilon mutants cannot be explained on the basis of interference with theta function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinkova A., Hervas C., Stukenberg P. T., Onrust R., O'Donnell M. E., Walker J. R. The Escherichia coli DNA polymerase III holoenzyme contains both products of the dnaX gene, tau and gamma, but only tau is essential. J Bacteriol. 1993 Sep;175(18):6018–6027. doi: 10.1128/jb.175.18.6018-6027.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomfield I. C., Vaughn V., Rest R. F., Eisenstein B. I. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol Microbiol. 1991 Jun;5(6):1447–1457. doi: 10.1111/j.1365-2958.1991.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Burton P., Holland I. B. Two pathways of division inhibition in UV-irradiated E. coli. Mol Gen Genet. 1983;190(1):128–132. doi: 10.1007/BF00330334. [DOI] [PubMed] [Google Scholar]
- Carter J. R., Franden M. A., Aebersold R., Kim D. R., McHenry C. S. Isolation, sequencing and overexpression of the gene encoding the theta subunit of DNA polymerase III holoenzyme. Nucleic Acids Res. 1993 Jul 11;21(14):3281–3286. doi: 10.1093/nar/21.14.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Knill-Jones J. W. Contribution of 3' leads to 5' exonuclease activity of DNA polymerase III holoenzyme from Escherichia coli to specificity. J Mol Biol. 1983 Apr 25;165(4):669–682. doi: 10.1016/s0022-2836(83)80273-3. [DOI] [PubMed] [Google Scholar]
- Foster P. L., Marinus M. G. Levels of epsilon, an essential replication subunit of Escherichia coli DNA polymerase III, are controlled by heat shock proteins. J Bacteriol. 1992 Dec;174(23):7509–7516. doi: 10.1128/jb.174.23.7509-7516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Sullivan A. D., Franklin S. B. Presence of the dnaQ-rnh divergent transcriptional unit on a multicopy plasmid inhibits induced mutagenesis in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3144–3151. doi: 10.1128/jb.171.6.3144-3151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagensee M. E., Timme T. L., Bryan S. K., Moses R. E. DNA polymerase III of Escherichia coli is required for UV and ethyl methanesulfonate mutagenesis. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4195–4199. doi: 10.1073/pnas.84.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath J. D., Perkins J. D., Sharma B., Weinstock G. M. NotI genomic cleavage map of Escherichia coli K-12 strain MG1655. J Bacteriol. 1992 Jan;174(2):558–567. doi: 10.1128/jb.174.2.558-567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., George J. Inducible sfi dependent division inhibition in Escherichia coli. Mol Gen Genet. 1980;177(4):629–636. doi: 10.1007/BF00272673. [DOI] [PubMed] [Google Scholar]
- Jaffé A., D'Ari R., Norris V. SOS-independent coupling between DNA replication and cell division in Escherichia coli. J Bacteriol. 1986 Jan;165(1):66–71. doi: 10.1128/jb.165.1.66-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonczyk P., Fijalkowska I., Ciesla Z. Overproduction of the epsilon subunit of DNA polymerase III counteracts the SOS mutagenic response of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9124–9127. doi: 10.1073/pnas.85.23.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X. P., Onrust R., O'Donnell M., Kuriyan J. Three-dimensional structure of the beta subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell. 1992 May 1;69(3):425–437. doi: 10.1016/0092-8674(92)90445-i. [DOI] [PubMed] [Google Scholar]
- Lancy E. D., Lifsics M. R., Kehres D. G., Maurer R. Isolation and characterization of mutants with deletions in dnaQ, the gene for the editing subunit of DNA polymerase III in Salmonella typhimurium. J Bacteriol. 1989 Oct;171(10):5572–5580. doi: 10.1128/jb.171.10.5572-5580.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancy E. D., Lifsics M. R., Munson P., Maurer R. Nucleotide sequences of dnaE, the gene for the polymerase subunit of DNA polymerase III in Salmonella typhimurium, and a variant that facilitates growth in the absence of another polymerase subunit. J Bacteriol. 1989 Oct;171(10):5581–5586. doi: 10.1128/jb.171.10.5581-5586.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifsics M. R., Lancy E. D., Jr, Maurer R. DNA replication defect in Salmonella typhimurium mutants lacking the editing (epsilon) subunit of DNA polymerase III. J Bacteriol. 1992 Nov;174(21):6965–6973. doi: 10.1128/jb.174.21.6965-6973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Harper J. E. Identification of the lexA gene product of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6147–6151. doi: 10.1073/pnas.76.12.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Scheuermann R. H., Echols H. Capacity of RecA protein to bind preferentially to UV lesions and inhibit the editing subunit (epsilon) of DNA polymerase III: a possible mechanism for SOS-induced targeted mutagenesis. Proc Natl Acad Sci U S A. 1986 Feb;83(3):619–623. doi: 10.1073/pnas.83.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki H., Kornberg A. Proofreading by DNA polymerase III of Escherichia coli depends on cooperative interaction of the polymerase and exonuclease subunits. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4389–4392. doi: 10.1073/pnas.84.13.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki H., Kornberg A. The polymerase subunit of DNA polymerase III of Escherichia coli. II. Purification of the alpha subunit, devoid of nuclease activities. J Biol Chem. 1985 Oct 25;260(24):12987–12992. [PubMed] [Google Scholar]
- Maki H., Maki S., Kornberg A. DNA Polymerase III holoenzyme of Escherichia coli. IV. The holoenzyme is an asymmetric dimer with twin active sites. J Biol Chem. 1988 May 15;263(14):6570–6578. [PubMed] [Google Scholar]
- Maurer R., Wong A. Dominant lethal mutations in the dnaB helicase gene of Salmonella typhimurium. J Bacteriol. 1988 Aug;170(8):3682–3688. doi: 10.1128/jb.170.8.3682-3688.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C. S. DNA polymerase III holoenzyme of Escherichia coli. Annu Rev Biochem. 1988;57:519–550. doi: 10.1146/annurev.bi.57.070188.002511. [DOI] [PubMed] [Google Scholar]
- Nomura T., Aiba H., Ishihama A. Transcriptional organization of the convergent overlapping dnaQ-rnh genes of Escherichia coli. J Biol Chem. 1985 Jun 10;260(11):7122–7125. [PubMed] [Google Scholar]
- O'Donnell M., Kuriyan J., Kong X. P., Stukenberg P. T., Onrust R. The sliding clamp of DNA polymerase III holoenzyme encircles DNA. Mol Biol Cell. 1992 Sep;3(9):953–957. doi: 10.1091/mbc.3.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan M., Lu C., Woodgate R., O'Donnell M., Goodman M. F., Echols H. Activity of the purified mutagenesis proteins UmuC, UmuD', and RecA in replicative bypass of an abasic DNA lesion by DNA polymerase III. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10777–10781. doi: 10.1073/pnas.89.22.10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassanfar M., Roberts J. W. Nature of the SOS-inducing signal in Escherichia coli. The involvement of DNA replication. J Mol Biol. 1990 Mar 5;212(1):79–96. doi: 10.1016/0022-2836(90)90306-7. [DOI] [PubMed] [Google Scholar]
- Scheuermann R. H., Echols H. A separate editing exonuclease for DNA replication: the epsilon subunit of Escherichia coli DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7747–7751. doi: 10.1073/pnas.81.24.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S. C., Maurer R. Requirements for bypass of UV-induced lesions in single-stranded DNA of bacteriophage phi X174 in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1251–1255. doi: 10.1073/pnas.88.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S., Maurer R. Simple phagemid-based system for generating allele replacements in Escherichia coli. J Bacteriol. 1993 Jul;175(13):4260–4262. doi: 10.1128/jb.175.13.4260-4262.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studwell-Vaughan P. S., O'Donnell M. Constitution of the twin polymerase of DNA polymerase III holoenzyme. J Biol Chem. 1991 Oct 15;266(29):19833–19841. [PubMed] [Google Scholar]
- Studwell-Vaughan P. S., O'Donnell M. DNA polymerase III accessory proteins. V. Theta encoded by holE. J Biol Chem. 1993 Jun 5;268(16):11785–11791. [PubMed] [Google Scholar]
- Studwell P. S., O'Donnell M. Processive replication is contingent on the exonuclease subunit of DNA polymerase III holoenzyme. J Biol Chem. 1990 Jan 15;265(2):1171–1178. [PubMed] [Google Scholar]
- Stukenberg P. T., Studwell-Vaughan P. S., O'Donnell M. Mechanism of the sliding beta-clamp of DNA polymerase III holoenzyme. J Biol Chem. 1991 Jun 15;266(17):11328–11334. [PubMed] [Google Scholar]
- Villani G., Boiteux S., Radman M. Mechanism of ultraviolet-induced mutagenesis: extent and fidelity of in vitro DNA synthesis on irradiated templates. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3037–3041. doi: 10.1073/pnas.75.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]