Abstract
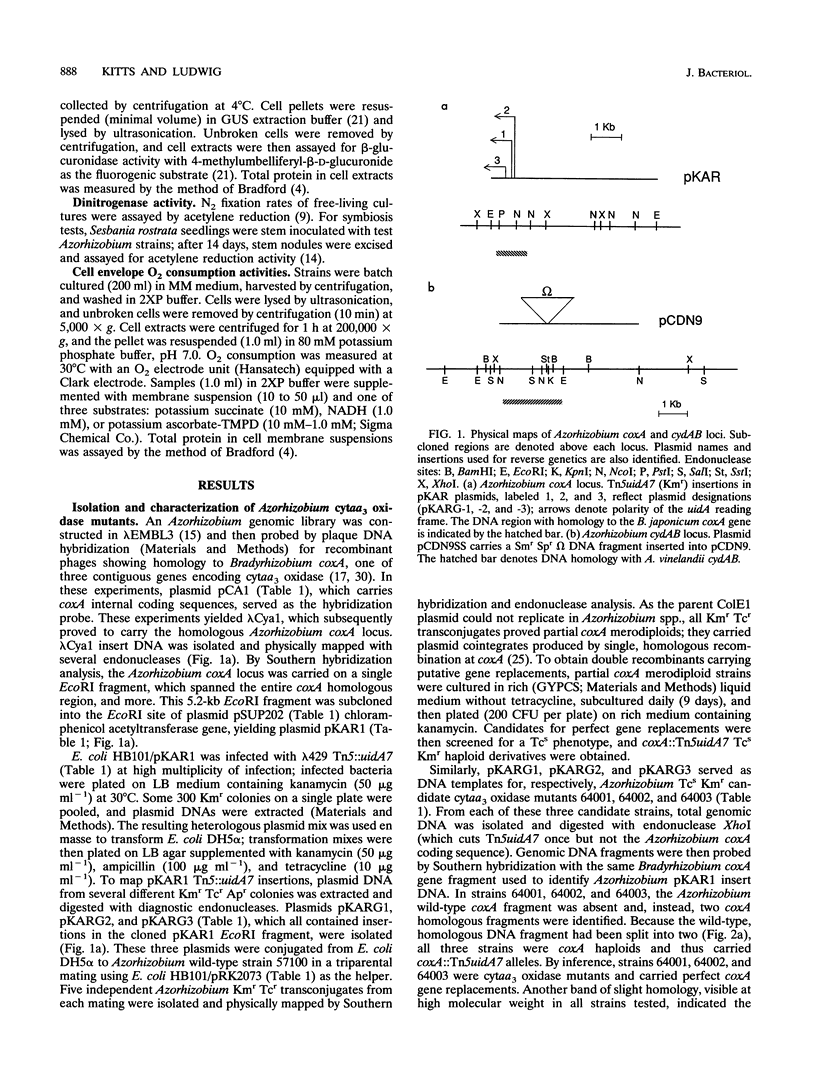
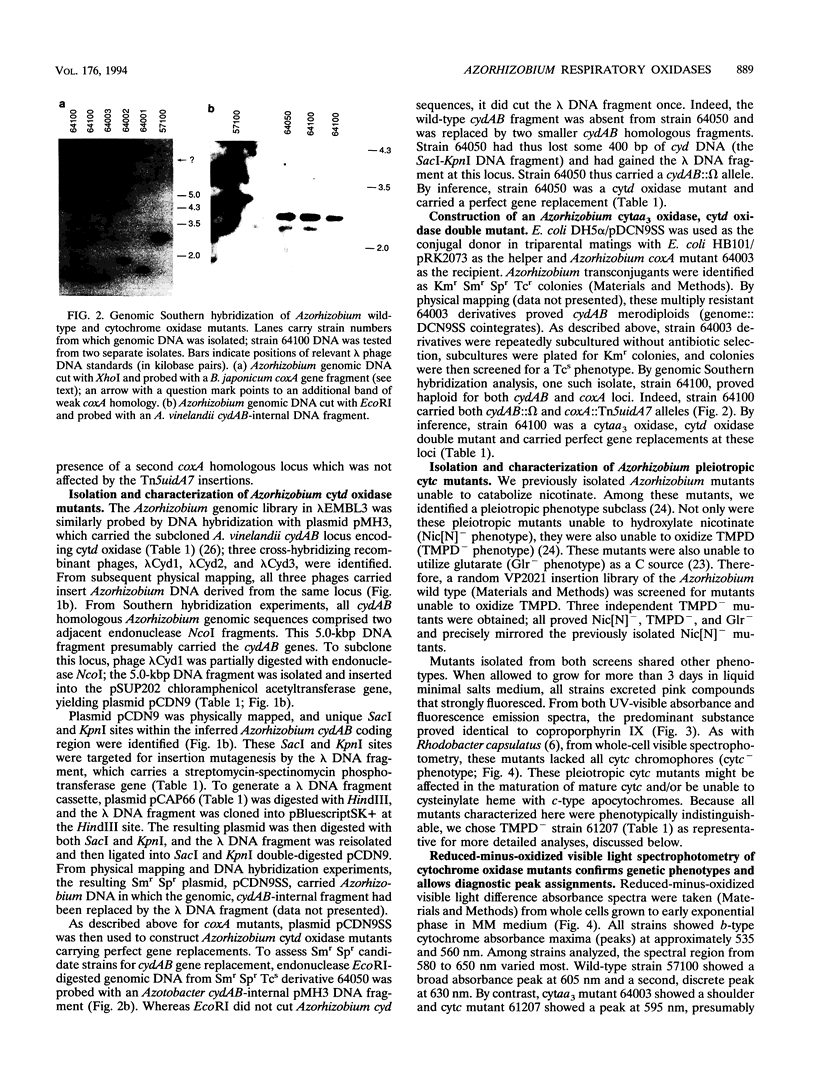
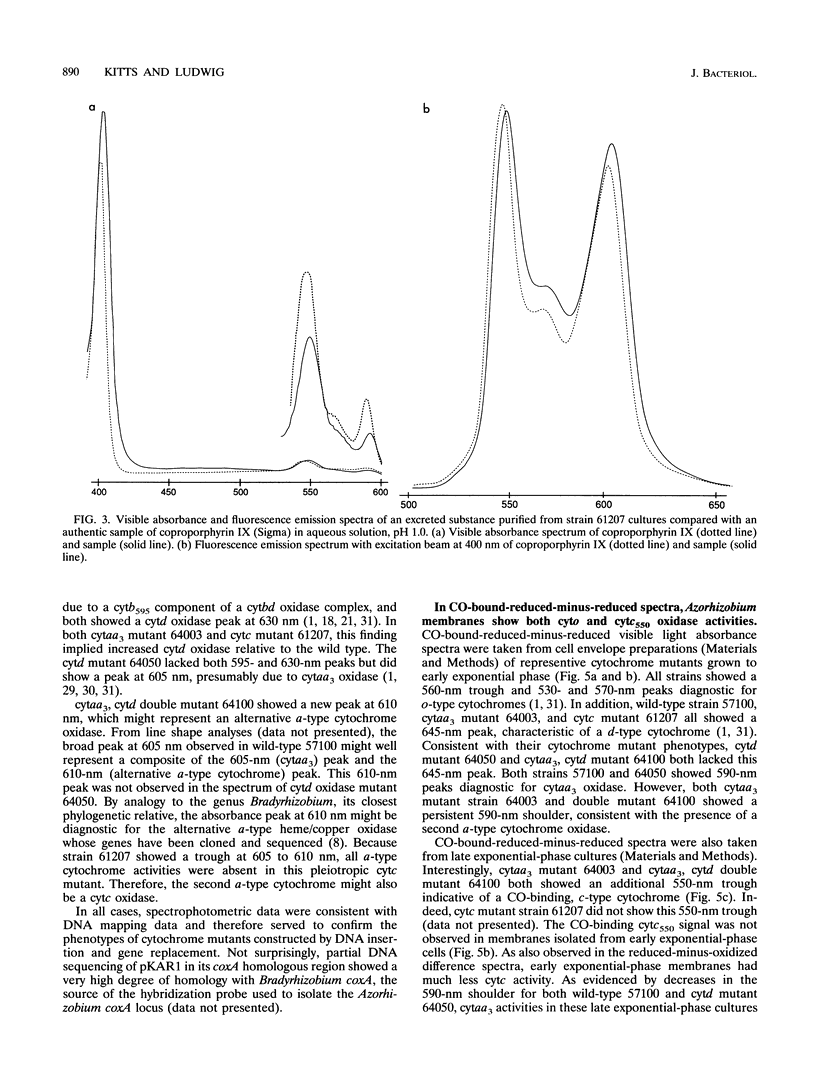
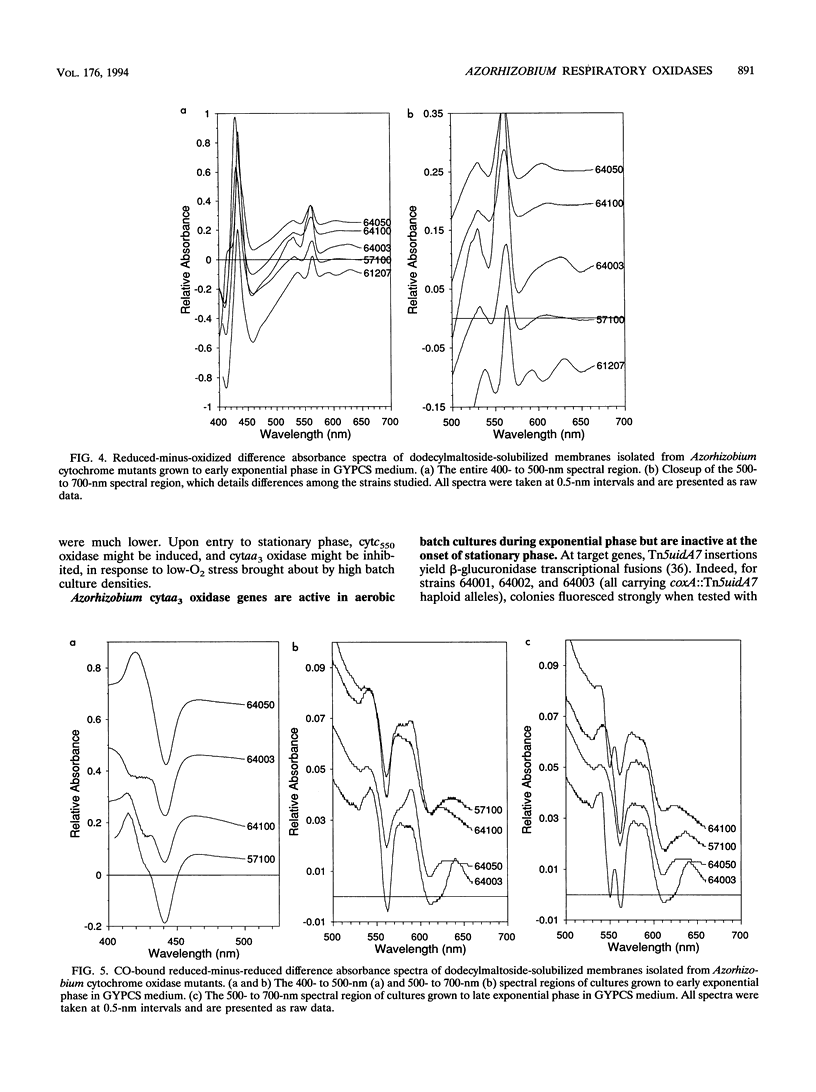
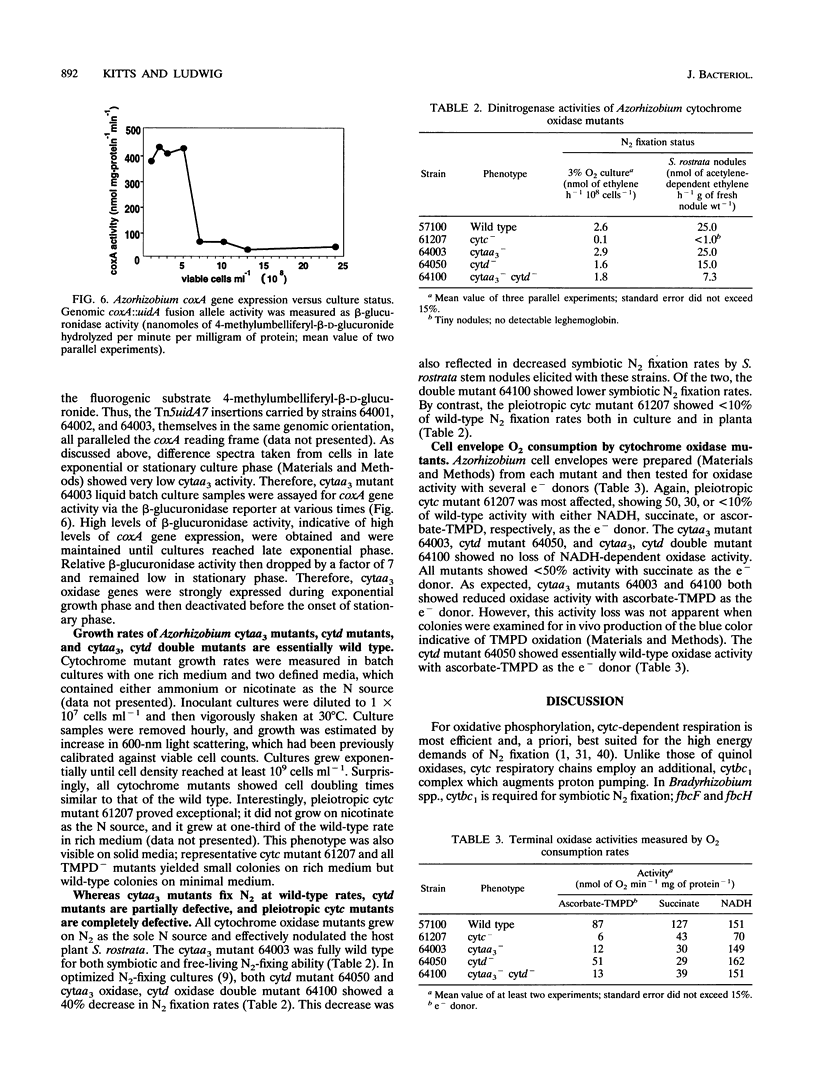
In culture, Azorhizobium caulinodans used at least four terminal oxidases, cytochrome aa3 (cytaa3), cytd, cyto, and a second a-type cytochrome, which together mediated general, respiratory electron (e-) transport to O2. To genetically dissect physiological roles for these various terminal oxidases, corresponding Azorhizobium apocytochrome genes were cloned, and three cytaa3 mutants, a cytd mutant, and a cytaa3, cytd double mutant were constructed by reverse genetics. These cytochrome oxidase mutants were tested for growth, oxidase activities, and N2 fixation properties both in culture and in symbiosis with the host plant Sesbania rostrata. The cytaa3 mutants grew normally, fixed N2 normally, and remained fully able to oxidize general respiratory e- donors (NADH, succinate) which utilize a cytc-dependent oxidase. By difference spectroscopy, a second, a-type cytochrome was detected in the cytaa3 mutants. This alternative a-type cytochrome (Amax = 610 nm) was also present in the wild type but was masked by bona fide cytaa3 (Amax = 605 nm). In late exponential-phase cultures, the cytaa3 mutants induced a new, membrane-bound, CO-binding cytc550, which also might serve as a cytc oxidase (a fifth terminal oxidase). The cloned Azorhizobium cytaa3 genes were strongly expressed during exponential growth but were deactivated prior to onset of stationary phase. Azorhizobium cytd mutants showed 40% lower N2 fixation rates in culture and in planta, but aerobic growth rates were wild type. The cytaa3, cytd double mutant showed 70% lower N2 fixation rates in planta. Pleiotropic cytc mutants were isolated by screening for strains unable to use N,N,N',N'-tetramethyl-p-phenylenediamine as a respiratory e- donor. These mutants synthesized no detectable cytc, excreted coproporphyrin, grew normally in aerobic minimal medium, grew poorly in rich medium, and fixed N2 poorly both in culture and in planta. Therefore, while aerobic growth was sustained by quinol oxidases alone, N2 fixation required cytc oxidase activities. Assuming that the terminal oxidases function as do their homologs in other bacteria, Azorhizobium respiration simultaneously employs both quinol and cytc oxidases. Because Azorhizobium terminal oxidase mutants were able to reformulate their terminal oxidase mix and grow more or less normally in aerobic culture, these terminal oxidases are somewhat degenerate. Its extensive terminal oxidase repertoire might allow Azorhizobium spp. to flourish in wide-ranging O2 environments.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. Bacterial electron transport chains. Annu Rev Biochem. 1988;57:101–132. doi: 10.1146/annurev.bi.57.070188.000533. [DOI] [PubMed] [Google Scholar]
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Biel S. W., Biel A. J. Isolation of a Rhodobacter capsulatus mutant that lacks c-type cytochromes and excretes porphyrins. J Bacteriol. 1990 Mar;172(3):1321–1326. doi: 10.1128/jb.172.3.1321-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott M., Bolliger M., Hennecke H. Genetic analysis of the cytochrome c-aa3 branch of the Bradyrhizobium japonicum respiratory chain. Mol Microbiol. 1990 Dec;4(12):2147–2157. doi: 10.1111/j.1365-2958.1990.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Bott M., Preisig O., Hennecke H. Genes for a second terminal oxidase in Bradyrhizobium japonicum. Arch Microbiol. 1992;158(5):335–343. doi: 10.1007/BF00245362. [DOI] [PubMed] [Google Scholar]
- Buckmiller L. M., Lapointe J. P., Ludwig R. A. Cloning of Azorhizobium caulinodans nicotinate catabolism genes and characterization of their importance in N2 fixation. J Bacteriol. 1991 Mar;173(6):2017–2025. doi: 10.1128/jb.173.6.2017-2025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepuri V., Lemieux L., Au D. C., Gennis R. B. The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J Biol Chem. 1990 Jul 5;265(19):11185–11192. [PubMed] [Google Scholar]
- Donald R. G., Ludwig R. A. Rhizobium sp. strain ORS571 ammonium assimilation and nitrogen fixation. J Bacteriol. 1984 Jun;158(3):1144–1151. doi: 10.1128/jb.158.3.1144-1151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R. G., Raymond C. K., Ludwig R. A. Vector insertion mutagenesis of Rhizobium sp. strain ORS571: direct cloning of mutagenized DNA sequences. J Bacteriol. 1985 Apr;162(1):317–323. doi: 10.1128/jb.162.1.317-323.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. N., Fang H., Lin R. J., Newton G., Mather M., Georgiou C. D., Gennis R. B. The nucleotide sequence of the cyd locus encoding the two subunits of the cytochrome d terminal oxidase complex of Escherichia coli. J Biol Chem. 1988 Sep 15;263(26):13138–13143. [PubMed] [Google Scholar]
- Hill S., Viollet S., Smith A. T., Anthony C. Roles for enteric d-type cytochrome oxidase in N2 fixation and microaerobiosis. J Bacteriol. 1990 Apr;172(4):2071–2078. doi: 10.1128/jb.172.4.2071-2078.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966 Mar 7;113(3):467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- Kelly M. J., Poole R. K., Yates M. G., Kennedy C. Cloning and mutagenesis of genes encoding the cytochrome bd terminal oxidase complex in Azotobacter vinelandii: mutants deficient in the cytochrome d complex are unable to fix nitrogen in air. J Bacteriol. 1990 Oct;172(10):6010–6019. doi: 10.1128/jb.172.10.6010-6019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts C. L., Lapointe J. P., Lam V. T., Ludwig R. A. Elucidation of the complete Azorhizobium nicotinate catabolism pathway. J Bacteriol. 1992 Dec;174(23):7791–7797. doi: 10.1128/jb.174.23.7791-7797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts C. L., Schaechter L. E., Rabin R. S., Ludwig R. A. Identification of cyclic intermediates in Azorhizobium caulinodans nicotinate catabolism. J Bacteriol. 1989 Jun;171(6):3406–3411. doi: 10.1128/jb.171.6.3406-3411.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshiri F., Chawla A., Maier R. J. Cloning, characterization, and expression in Escherichia coli of the genes encoding the cytochrome d oxidase complex from Azotobacter vinelandii. J Bacteriol. 1991 Oct;173(19):6230–6241. doi: 10.1128/jb.173.19.6230-6241.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal C. S., van Berkum P., Sadowsky M. J., Keister D. L. Cytochrome mutants of bradyrhizobium induced by transposon tn5. Plant Physiol. 1989 Jun;90(2):553–559. doi: 10.1104/pp.90.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Kirshbom P. M., Maier R. J. Tn5-induced cytochrome mutants of Bradyrhizobium japonicum: effects of the mutations on cells grown symbiotically and in culture. J Bacteriol. 1987 Mar;169(3):1089–1094. doi: 10.1128/jb.169.3.1089-1094.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'brian M. R., Maier R. J. Isolation of a cytochrome aa(3) gene from Bradyrhizobium japonicum. Proc Natl Acad Sci U S A. 1987 May;84(10):3219–3223. doi: 10.1073/pnas.84.10.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S. C., Anderson P., Wickens M. P. Splicing of a C. elegans myosin pre-mRNA in a human nuclear extract. Nucleic Acids Res. 1990 Jan 11;18(1):143–149. doi: 10.1093/nar/18.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- Preisig O., Anthamatten D., Hennecke H. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3309–3313. doi: 10.1073/pnas.90.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitio M., Jalli T., Saraste M. Isolation and analysis of the genes for cytochrome c oxidase in Paracoccus denitrificans. EMBO J. 1987 Sep;6(9):2825–2833. doi: 10.1002/j.1460-2075.1987.tb02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach S., Loferer H., Acuña G., Appleby C. A., Hennecke H. Cloning, sequencing and mutational analysis of the cytochrome c552 gene (cycB) from Bradyrhizobium japonicum strain 110. FEMS Microbiol Lett. 1991 Oct 1;67(2):145–152. doi: 10.1016/0378-1097(91)90345-b. [DOI] [PubMed] [Google Scholar]
- Sharma S. B., Signer E. R. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in planta revealed by transposon Tn5-gusA. Genes Dev. 1990 Mar;4(3):344–356. doi: 10.1101/gad.4.3.344. [DOI] [PubMed] [Google Scholar]
- Smith A., Hill S., Anthony C. The purification, characterization and role of the d-type cytochrome oxidase of Klebsiella pneumoniae during nitrogen fixation. J Gen Microbiol. 1990 Jan;136(1):171–180. doi: 10.1099/00221287-136-1-171. [DOI] [PubMed] [Google Scholar]
- Soberón M., Williams H. D., Poole R. K., Escamilla E. Isolation of a Rhizobium phaseoli cytochrome mutant with enhanced respiration and symbiotic nitrogen fixation. J Bacteriol. 1989 Jan;171(1):465–472. doi: 10.1128/jb.171.1.465-472.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöny-Meyer L., Stax D., Hennecke H. An unusual gene cluster for the cytochrome bc1 complex in Bradyrhizobium japonicum and its requirement for effective root nodule symbiosis. Cell. 1989 May 19;57(4):683–697. doi: 10.1016/0092-8674(89)90137-2. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Sadowsky M. J., Keister D. L. Characterization of cytochromes c550 and c555 from Bradyrhizobium japonicum: cloning, mutagenesis, and sequencing of the c555 gene (cycC). J Bacteriol. 1991 Dec;173(24):7887–7895. doi: 10.1128/jb.173.24.7887-7895.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. Biochemical and biophysical properties of cytochrome o of Azotobacter vinelandii. Biochim Biophys Acta. 1986 Mar 12;848(3):342–351. doi: 10.1016/0005-2728(86)90209-4. [DOI] [PubMed] [Google Scholar]
- de Vries W., Stam H., Stouthamer A. H. Hydrogen oxidation and nitrogen fixation in rhizobia, with special attention focused on strain ORS 571. Antonie Van Leeuwenhoek. 1984;50(5-6):505–524. doi: 10.1007/BF02386223. [DOI] [PubMed] [Google Scholar]