Abstract
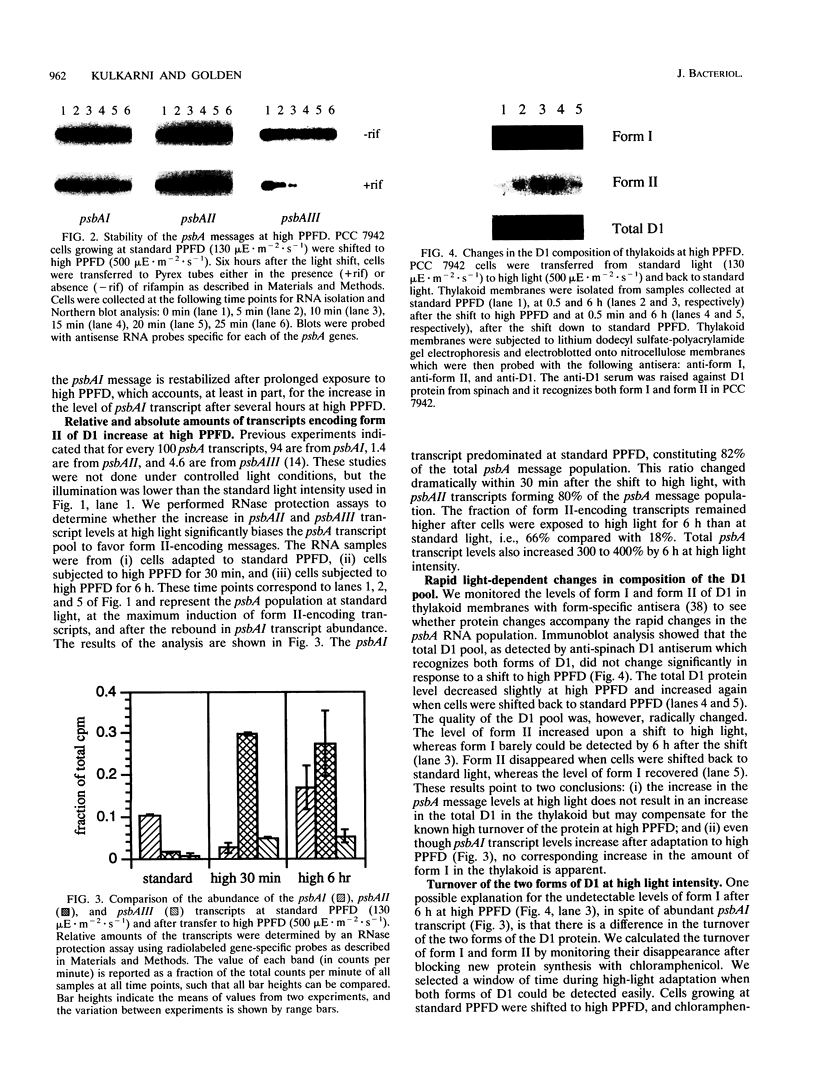
The three psbA genes in the cyanobacterium Synechococcus sp. strain PCC 7942 encode two distinct forms of the D1 protein of photosystem II. The psbAI message, which encodes form I, dominates the psbA transcript pool at low to moderate light intensities; however, exposure to high light triggers a response in which the psbAI message is actively degraded while psbAII and psbAIII, which encode form II, are transcriptionally induced. We addressed whether these changes result from a generalized stress response and examined the consequence of light-responsive psbA regulation on the composition of D1 in thylakoid membranes. Heat shock and oxidative stress had some effect on levels of the three psbA transcripts but did not produce the responses generated by an increase in light intensity. Prolonged exposure to high light (24-h time course) was characterized by elevated levels of all psbA transcripts through maintenance of high levels of psbAII and psbAIII messages and a rebound of the psbAI transcript after its initial decline. Form II-encoding transcripts were enriched relative to those encoding form I at all high-light time points. Form II replaced form I in the thylakoid membrane at high light despite an abundance of psbAI transcript at later time points; this may be explained by the observed faster turnover of form I than form II in the membrane. We propose that form II is less susceptible to damage at high light and that this qualitative alteration, coupled with increased turnover of D1, protects the cells from photoinhibition.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouyoub A., Vernotte C., Astier C. Functional analysis of the two homologous psbA gene copies in Synechocystis PCC 6714 and PCC 6803. Plant Mol Biol. 1993 Jan;21(2):249–258. doi: 10.1007/BF00019941. [DOI] [PubMed] [Google Scholar]
- Brusslan J., Haselkorn R. Resistance to the photosystem II herbicide diuron is dominant to sensitivity in the cyanobacterium Synechococcus sp. PCC7942. EMBO J. 1989 Apr;8(4):1237–1245. doi: 10.1002/j.1460-2075.1989.tb03497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos S. A., Golden S. S. Light-regulated expression of the psbD gene family in Synechococcus sp. strain PCC 7942: evidence for the role of duplicated psbD genes in cyanobacteria. Mol Gen Genet. 1992 Mar;232(2):221–230. doi: 10.1007/BF00280000. [DOI] [PubMed] [Google Scholar]
- Bustos S. A., Schaefer M. R., Golden S. S. Different and rapid responses of four cyanobacterial psbA transcripts to changes in light intensity. J Bacteriol. 1990 Apr;172(4):1998–2004. doi: 10.1128/jb.172.4.1998-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. K., Soitamo A., Gustafsson P., Oquist G. Rapid interchange between two distinct forms of cyanobacterial photosystem II reaction-center protein D1 in response to photoinhibition. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9973–9977. doi: 10.1073/pnas.90.21.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991 Dec;55(4):561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruissem W. Chloroplast gene expression: how plants turn their plastids on. Cell. 1989 Jan 27;56(2):161–170. doi: 10.1016/0092-8674(89)90889-1. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Chlorophyll-protein organization of membranes from the cyanobacterium Anacystis nidulans. Arch Biochem Biophys. 1983 Jan;220(1):155–166. doi: 10.1016/0003-9861(83)90396-x. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Jansson C., Debus R. J., Osiewacz H. D., Gurevitz M., McIntosh L. Construction of an Obligate Photoheterotrophic Mutant of the Cyanobacterium Synechocystis 6803 : Inactivation of the psbA Gene Family. Plant Physiol. 1987 Dec;85(4):1021–1025. doi: 10.1104/pp.85.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Strayer C. A., Kulkarni R. D., Taylor W., Ishiura M., Golden S. S., Johnson C. H. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5672–5676. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R. D., Schaefer M. R., Golden S. S. Transcriptional and posttranscriptional components of psbA response to high light intensity in Synechococcus sp. strain PCC 7942. J Bacteriol. 1992 Jun;174(11):3775–3781. doi: 10.1128/jb.174.11.3775-3781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Golden S. S. Enhancer activity of light-responsive regulatory elements in the untranslated leader regions of cyanobacterial psbA genes. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11678–11682. doi: 10.1073/pnas.90.24.11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A., Jansson C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol. 1989 Dec;13(6):693–700. doi: 10.1007/BF00016024. [DOI] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Arntzen C. J. Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast membranes. J Cell Biol. 1984 Aug;99(2):481–485. doi: 10.1083/jcb.99.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Hirschberg J. Light-dependent degradation of the Q(B)-protein in isolated pea thylakoids. EMBO J. 1985 Jul;4(7):1655–1659. doi: 10.1002/j.1460-2075.1985.tb03833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad N., Amir-Shapira D., Koike H., Inoue Y., Ohad I., Hirschberg J. Amino acid substitutions in the D1 protein of photosystem II affect QB- stabilization and accelerate turnover of D1. Z Naturforsch C. 1990 May;45(5):402–407. doi: 10.1515/znc-1990-0515. [DOI] [PubMed] [Google Scholar]
- Schaefer M. R., Golden S. S. Differential expression of members of a cyanobacterial psbA gene family in response to light. J Bacteriol. 1989 Jul;171(7):3973–3981. doi: 10.1128/jb.171.7.3973-3981.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M. R., Golden S. S. Light availability influences the ratio of two forms of D1 in cyanobacterial thylakoids. J Biol Chem. 1989 May 5;264(13):7412–7417. [PubMed] [Google Scholar]
- Schuster G., Timberg R., Ohad I. Turnover of thylakoid photosystem II proteins during photoinhibition of Chlamydomonas reinhardtii. Eur J Biochem. 1988 Nov 1;177(2):403–410. doi: 10.1111/j.1432-1033.1988.tb14389.x. [DOI] [PubMed] [Google Scholar]
- Vrba J. M., Curtis S. E. Characterization of a four-member psbA gene family from the cyanobacterium Anabaena PCC 7120. Plant Mol Biol. 1990 Jan;14(1):81–92. doi: 10.1007/BF00015657. [DOI] [PubMed] [Google Scholar]
- Webb R., Reddy K. J., Sherman L. A. Regulation and sequence of the Synechococcus sp. strain PCC 7942 groESL operon, encoding a cyanobacterial chaperonin. J Bacteriol. 1990 Sep;172(9):5079–5088. doi: 10.1128/jb.172.9.5079-5088.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]