Abstract
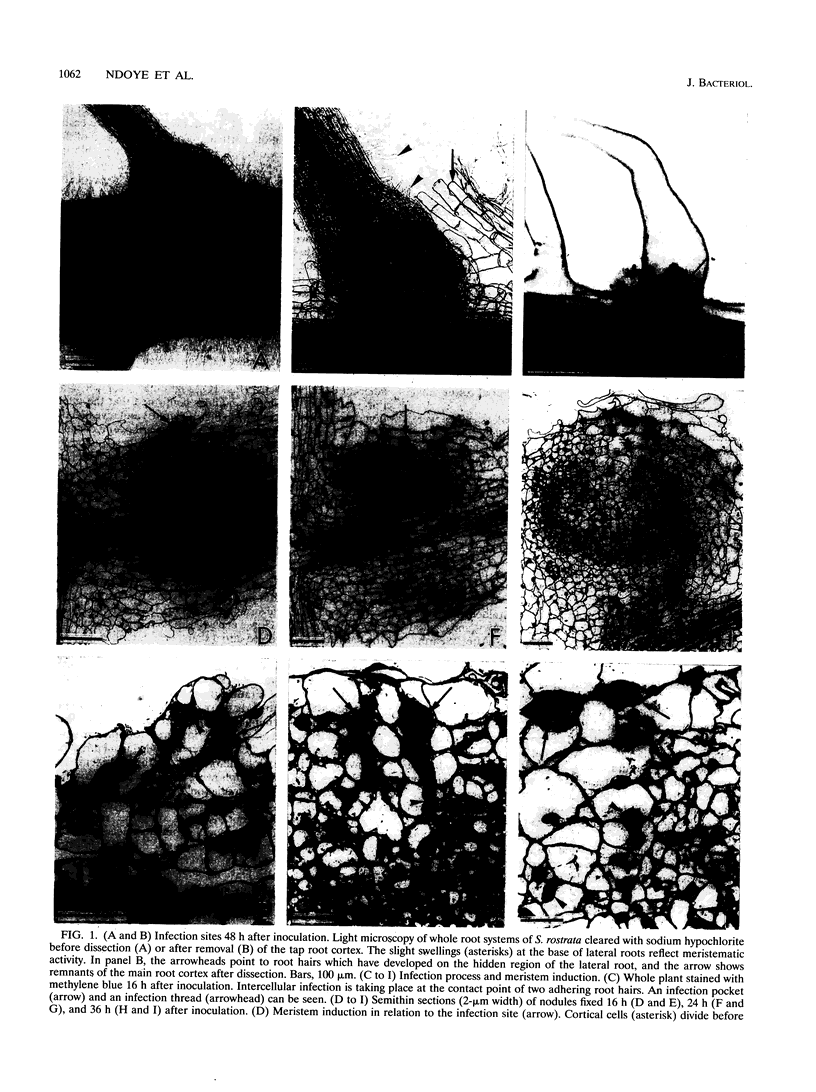
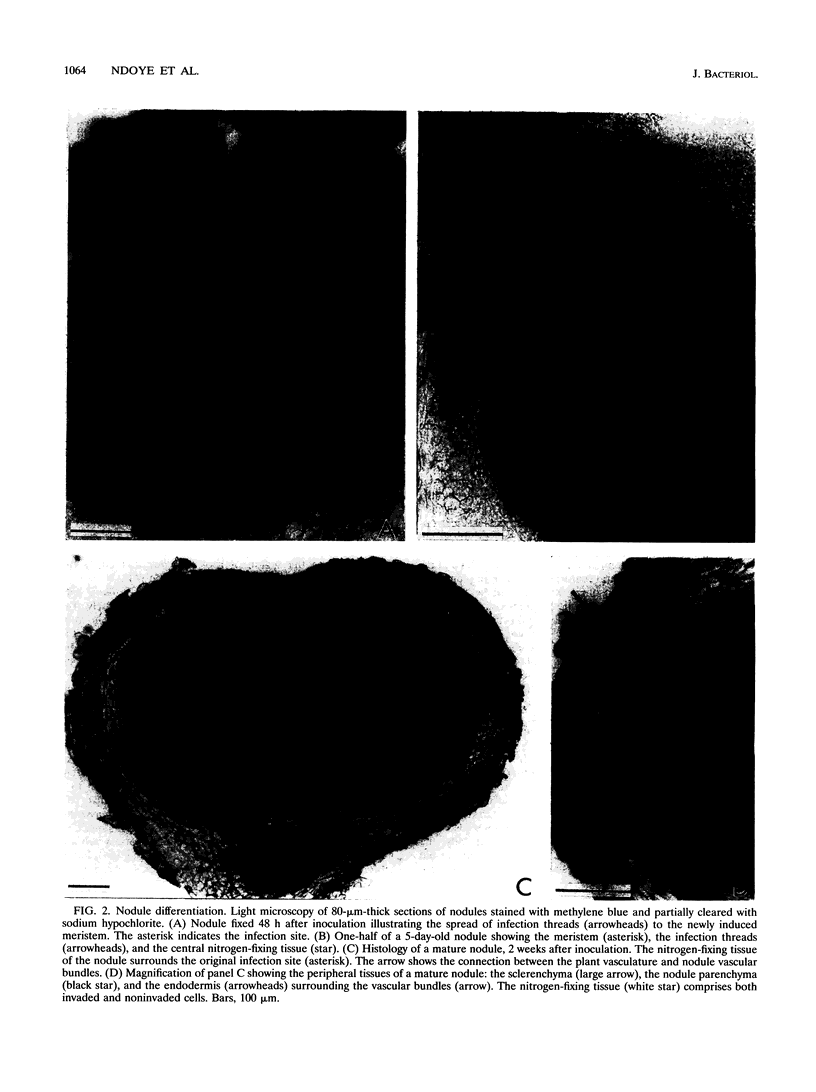
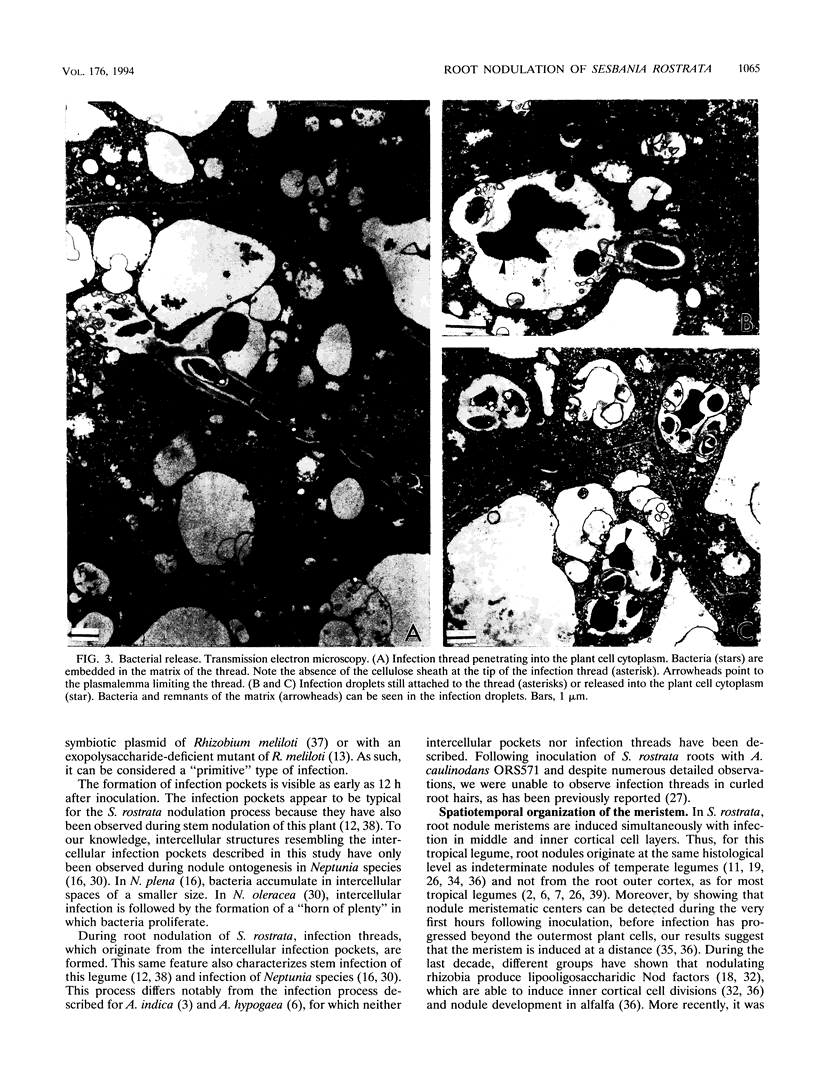
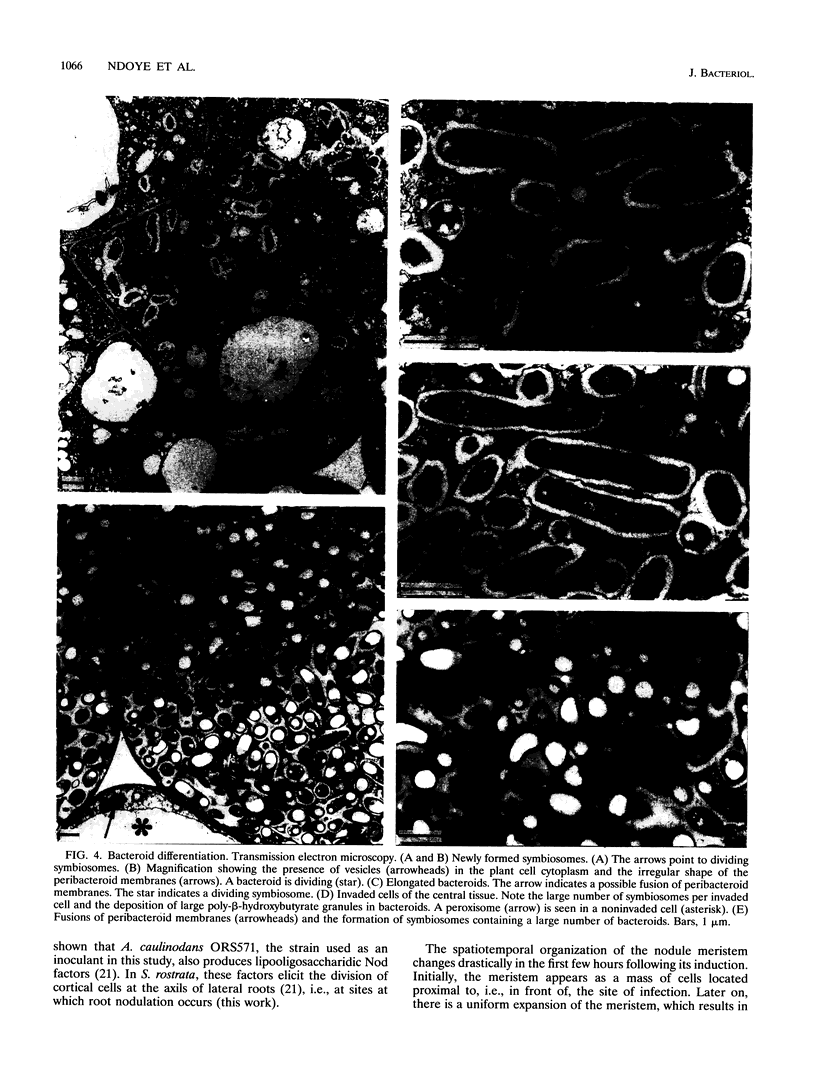
The tropical legume Sesbania rostrata can be nodulated by Azorhizobium caulinodans on both its stem and its root system. Here we investigate in detail the process of root nodulation and show that nodules develop exclusively at the base of secondary roots. Intercellular infection leads to the formation of infection pockets, which then give rise to infection threads. Concomitantly with infection, cortical cells of the secondary roots dedifferentiate, forming a meristem which has an "open-basket" configuration and which surrounds the initial infection site. Bacteria are released from the tips of infection threads into plant cells via "infection droplets," each containing several bacteria. Initially, nodule differentiation is comparable to that of indeterminate nodules, with the youngest meristematic cells being located at the periphery and the nitrogen-fixing cells being located at the nodule center. Because of the peculiar form of the meristem, Sesbania root nodules develop uniformly around a central axis. Nitrogen fixation is detected as early as 3 days following inoculation, while the nodule meristem is still active. Two weeks after inoculation, meristematic activity ceases, and nodules then show the typical histology of determinate nodules. Thus, root nodule organogenesis in S. rostrata appears to be intermediate between indeterminate and determinate types.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin N. J. Development of the legume root nodule. Annu Rev Cell Biol. 1991;7:191–226. doi: 10.1146/annurev.cb.07.110191.001203. [DOI] [PubMed] [Google Scholar]
- Creel S. R., Monfort S. L., Wildt D. E., Waser P. M. Spontaneous lactation is an adaptive result of pseudopregnancy. Nature. 1991 Jun 20;351(6328):660–662. doi: 10.1038/351660a0. [DOI] [PubMed] [Google Scholar]
- Dénarié J., Debellé F., Rosenberg C. Signaling and host range variation in nodulation. Annu Rev Microbiol. 1992;46:497–531. doi: 10.1146/annurev.mi.46.100192.002433. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Huber J. D., Parker F., Odland G. F. A basic fuchsin and alkalinized methylene blue rapid stain for epoxy-embedded tissue. Stain Technol. 1968 Mar;43(2):83–87. doi: 10.3109/10520296809115048. [DOI] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Mergaert P., Van Montagu M., Promé J. C., Holsters M. Three unusual modifications, a D-arabinosyl, an N-methyl, and a carbamoyl group, are present on the Nod factors of Azorhizobium caulinodans strain ORS571. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1551–1555. doi: 10.1073/pnas.90.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nap J. P., Bisseling T. Developmental biology of a plant-prokaryote symbiosis: the legume root nodule. Science. 1990 Nov 16;250(4983):948–954. doi: 10.1126/science.250.4983.948. [DOI] [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. G., Lyttleton P., Bullivant S., Grayston G. F. Membranes in lupin root nodules. I. The role of Golgi bodies in the biogenesis of infection threads and peribacteroid membranes. J Cell Sci. 1978 Apr;30:129–149. doi: 10.1242/jcs.30.1.129. [DOI] [PubMed] [Google Scholar]
- Spaink H. P., Sheeley D. M., van Brussel A. A., Glushka J., York W. S., Tak T., Geiger O., Kennedy E. P., Reinhold V. N., Lugtenberg B. J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991 Nov 14;354(6349):125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Truchet G., Rosenberg C., Vasse J., Julliot J. S., Camut S., Denarie J. Transfer of Rhizobium meliloti pSym genes into Agrobacterium tumefaciens: host-specific nodulation by atypical infection. J Bacteriol. 1984 Jan;157(1):134–142. doi: 10.1128/jb.157.1.134-142.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Dreyfus B. L., Schmidt E. L. Initial stages in the morphogenesis of nitrogen-fixing stem nodules of Sesbania rostrata. J Bacteriol. 1983 Nov;156(2):888–897. doi: 10.1128/jb.156.2.888-897.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse J., de Billy F., Camut S., Truchet G. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol. 1990 Aug;172(8):4295–4306. doi: 10.1128/jb.172.8.4295-4306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]