Abstract
A combined physical and genetic map of the Serpulina hyodysenteriae B78T genome was constructed by using pulsed-field gel electrophoresis and DNA blot hybridizations. The S. hyodysenteriae genome is a single circular chromosome about 3.2 Mb in size. The physical map of the chromosome was constructed with the restriction enzymes BssHII, EclXI, NotI, SalI, and SmaI. The physical map was used to constructed a linkage map for genes encoding rRNA, flagellum subunit proteins, DNA gyrase, NADH oxidase, and three distinct hemolysins. Several flaB2-related loci, encoding core flagellum subunit proteins, were detected and are dispersed around the chromosome. The rRNA gene organization in S. hyodysenteriae is unusual. S. hyodysenteriae has one gene each for 5S (rrf), 16S (rrs), and 23S (rrl) rRNAs. The rrf and rrl genes are closely linked (within 5 kb), while the rrs gene is about 860 kb from the other two rRNA genes. Using a probe for the S. hyodysenteriae gyrA gene, we identified a possible location for the chromosomal replication origin. The size and genetic organization of the S. hyodysenteriae chromosome are different from those of previously characterized spirochetes.
Full text
PDF

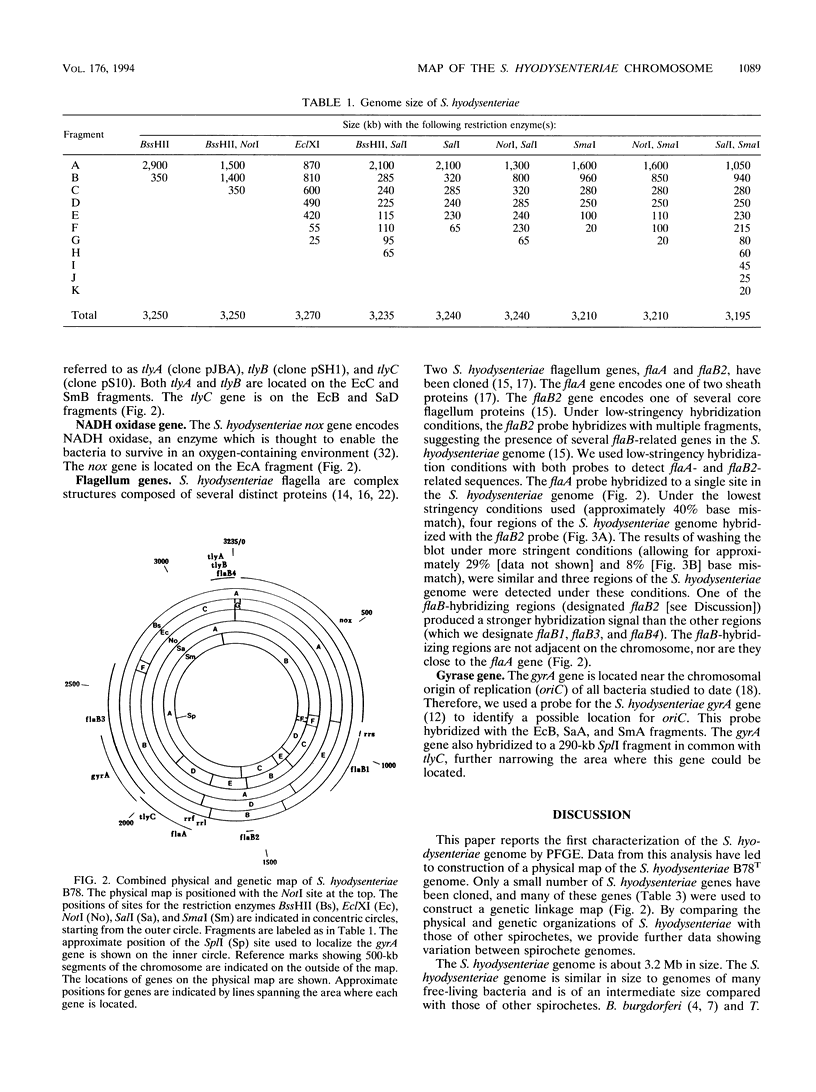
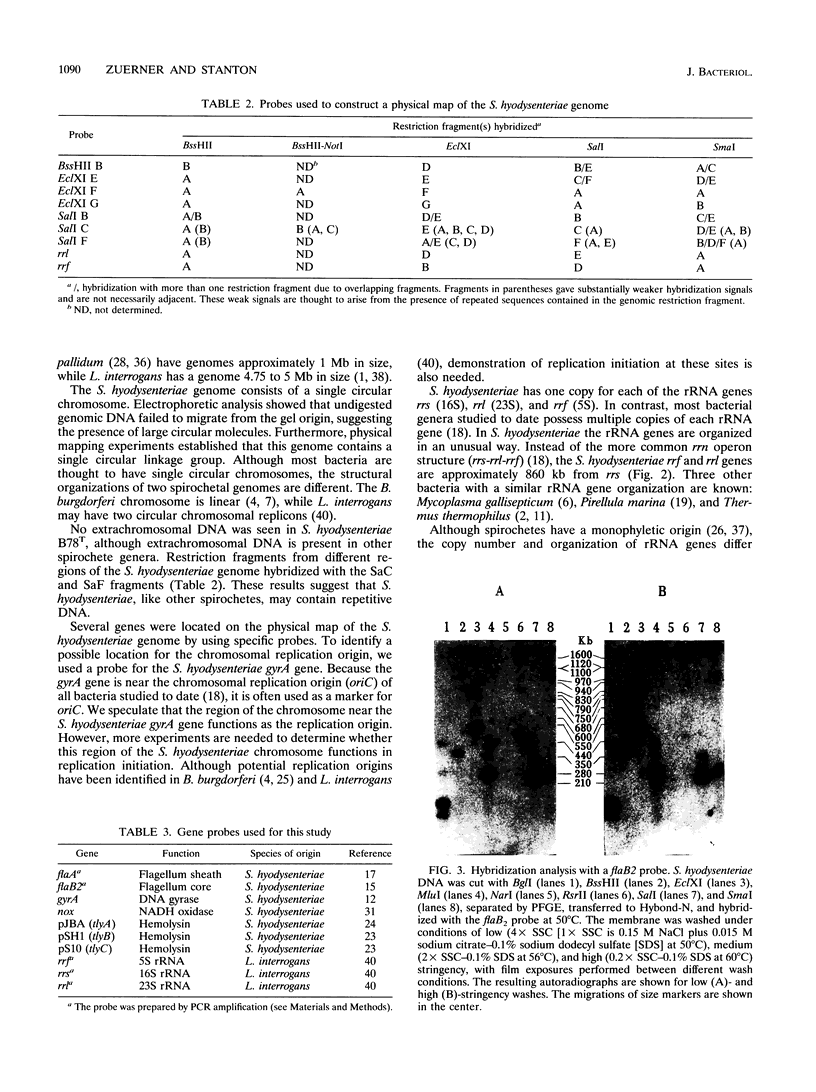


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baril C., Herrmann J. L., Richaud C., Margarita D., Girons I. S. Scattering of the rRNA genes on the physical map of the circular chromosome of Leptospira interrogans serovar icterohaemorrhagiae. J Bacteriol. 1992 Dec;174(23):7566–7571. doi: 10.1128/jb.174.23.7566-7571.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K. M., Bergquist P. L. Genomic restriction map of the extremely thermophilic bacterium Thermus thermophilus HB8. J Bacteriol. 1993 Jan;175(1):103–110. doi: 10.1128/jb.175.1.103-110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahamsha B., Greenberg E. P. Cloning and sequence analysis of flaA, a gene encoding a Spirochaeta aurantia flagellar filament surface antigen. J Bacteriol. 1989 Mar;171(3):1692–1697. doi: 10.1128/jb.171.3.1692-1697.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., Huang W. M. Linear chromosomal physical and genetic map of Borrelia burgdorferi, the Lyme disease agent. Mol Microbiol. 1993 May;8(5):967–980. doi: 10.1111/j.1365-2958.1993.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Champion C. I., Miller J. N., Lovett M. A., Blanco D. R. Cloning, sequencing, and expression of two class B endoflagellar genes of Treponema pallidum subsp. pallidum encoding the 34.5- and 31.0-kilodalton proteins. Infect Immun. 1990 Jun;58(6):1697–1704. doi: 10.1128/iai.58.6.1697-1704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Finch L. R. Novel arrangement of rRNA genes in Mycoplasma gallisepticum: separation of the 16S gene of one set from the 23S and 5S genes. J Bacteriol. 1989 May;171(5):2876–2878. doi: 10.1128/jb.171.5.2876-2878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B. E., MacDougall J., Saint Girons I. Physical map of the linear chromosome of the bacterium Borrelia burgdorferi 212, a causative agent of Lyme disease, and localization of rRNA genes. J Bacteriol. 1992 Jun;174(11):3766–3774. doi: 10.1128/jb.174.11.3766-3774.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M., Masuzawa T., Okuzako N., Mifuchi I., Yanagihara Y. Linkage of ribosomal RNA genes in Leptospira. Microbiol Immunol. 1990;34(7):565–573. doi: 10.1111/j.1348-0421.1990.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Okuzako N., Mifuchi I., Arimitsu Y., Seki M. Organization of the ribosomal RNA genes in Treponema phagedenis and Treponema pallidum. Microbiol Immunol. 1992;36(2):161–167. doi: 10.1111/j.1348-0421.1992.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Sohnaka M. Tandem repeat of the 23S and 5S ribosomal RNA genes in Borrelia burgdorferi, the etiological agent of Lyme disease. Biochem Biophys Res Commun. 1992 Mar 31;183(3):952–957. doi: 10.1016/s0006-291x(05)80282-7. [DOI] [PubMed] [Google Scholar]
- Fukunaga M., Yanagihara Y., Sohnaka M. The 23S/5S ribosomal RNA genes (rrl/rrf) are separate from the 16S ribosomal RNA gene (rrs) in Borrelia burgdorferi, the aetiological agent of Lyme disease. J Gen Microbiol. 1992 May;138(5):871–877. doi: 10.1099/00221287-138-5-871. [DOI] [PubMed] [Google Scholar]
- Hartmann R. K., Erdmann V. A. Thermus thermophilus 16S rRNA is transcribed from an isolated transcription unit. J Bacteriol. 1989 Jun;171(6):2933–2941. doi: 10.1128/jb.171.6.2933-2941.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs R. D., Hanke J. H., Guzman-Verduzco L. M., Newport G., Agabian N., Norgard M. V., Lukehart S. A., Radolf J. D. Molecular cloning and DNA sequence analysis of the 37-kilodalton endoflagellar sheath protein gene of Treponema pallidum. Infect Immun. 1989 Nov;57(11):3403–3411. doi: 10.1128/iai.57.11.3403-3411.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. A., Sellwood R., Lemcke R. M., Burrows M. R., Lysons R. J. Analysis of the axial filaments of Treponema hyodysenteriae by SDS-PAGE and immunoblotting. J Gen Microbiol. 1989 Jun;135(6):1625–1632. doi: 10.1099/00221287-135-6-1625. [DOI] [PubMed] [Google Scholar]
- Koopman M. B., Baats E., de Leeuw O. S., van der Zeijst B. A., Kusters J. G. Molecular analysis of a flagellar core protein gene of Serpulina (Treponema) hyodysenteriae. J Gen Microbiol. 1993 Aug;139(8):1701–1706. doi: 10.1099/00221287-139-8-1701. [DOI] [PubMed] [Google Scholar]
- Koopman M. B., Baats E., van Vorstenbosch C. J., van der Zeijst B. A., Kusters J. G. The periplasmic flagella of Serpulina (Treponema) hyodysenteriae are composed of two sheath proteins and three core proteins. J Gen Microbiol. 1992 Dec;138(12):2697–2706. doi: 10.1099/00221287-138-12-2697. [DOI] [PubMed] [Google Scholar]
- Koopman M. B., de Leeuw O. S., van der Zeijst B. M., Kusters J. G. Cloning and DNA sequence analysis of a Serpulina (Treponema) hyodysenteriae gene encoding a periplasmic flagellar sheath protein. Infect Immun. 1992 Jul;60(7):2920–2925. doi: 10.1128/iai.60.7.2920-2925.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawiec S., Riley M. Organization of the bacterial chromosome. Microbiol Rev. 1990 Dec;54(4):502–539. doi: 10.1128/mr.54.4.502-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesack W., Stackebrandt E. Evidence for unlinked rrn operons in the Planctomycete Pirellula marina. J Bacteriol. 1989 Sep;171(9):5025–5030. doi: 10.1128/jb.171.9.5025-5030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberger R. J., Slivienski L. L., Yelton D. B., Charon N. W. Molecular genetic analysis of a class B periplasmic-flagellum gene of Treponema phagedenis. J Bacteriol. 1992 Oct;174(20):6404–6410. doi: 10.1128/jb.174.20.6404-6410.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H., Harris D. L. Genetics of Treponema: characterization of Treponema hyodysenteriae and its relationship to Treponema pallidum. Infect Immun. 1978 Dec;22(3):736–739. doi: 10.1128/iai.22.3.736-739.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. P., Toivio-Kinnucan M., Wu G., Wilt G. R. Ultrastructural and electrophoretic analysis of Treponema hyodysenteriae axial filaments. Am J Vet Res. 1988 Jun;49(6):786–789. [PubMed] [Google Scholar]
- Muir S., Koopman M. B., Libby S. J., Joens L. A., Heffron F., Kusters J. G. Cloning and expression of a Serpula (Treponema) hyodysenteriae hemolysin gene. Infect Immun. 1992 Feb;60(2):529–535. doi: 10.1128/iai.60.2.529-535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old I. G., MacDougall J., Saint Girons I., Davidson B. E. Mapping of genes on the linear chromosome of the bacterium Borrelia burgdorferi: possible locations for its origin of replication. FEMS Microbiol Lett. 1992 Dec 1;78(2-3):245–250. doi: 10.1016/0378-1097(92)90034-l. [DOI] [PubMed] [Google Scholar]
- Paster B. J., Dewhirst F. E., Weisburg W. G., Tordoff L. A., Fraser G. J., Hespell R. B., Stanton T. B., Zablen L., Mandelco L., Woese C. R. Phylogenetic analysis of the spirochetes. J Bacteriol. 1991 Oct;173(19):6101–6109. doi: 10.1128/jb.173.19.6101-6109.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk R. H., Simon M. I., Barbour A. G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985 Nov 21;318(6043):257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- Saint Girons I., Norris S. J., Göbel U., Meyer J., Walker E. M., Zuerner R. Genome structure of spirochetes. Res Microbiol. 1992 Jul-Aug;143(6):615–621. doi: 10.1016/0923-2508(92)90119-9. [DOI] [PubMed] [Google Scholar]
- Schwartz J. J., Gazumyan A., Schwartz I. rRNA gene organization in the Lyme disease spirochete, Borrelia burgdorferi. J Bacteriol. 1992 Jun;174(11):3757–3765. doi: 10.1128/jb.174.11.3757-3765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. B., Jensen N. S., Casey T. A., Tordoff L. A., Dewhirst F. E., Paster B. J. Reclassification of Treponema hyodysenteriae and Treponema innocens in a new genus, Serpula gen. nov., as Serpula hyodysenteriae comb. nov. and Serpula innocens comb. nov. Int J Syst Bacteriol. 1991 Jan;41(1):50–58. doi: 10.1099/00207713-41-1-50. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Jensen N. S. Purification and characterization of NADH oxidase from Serpulina (Treponema) hyodysenteriae. J Bacteriol. 1993 May;175(10):2980–2987. doi: 10.1128/jb.175.10.2980-2987.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. B. Proposal to change the genus designation Serpula to Serpulina gen. nov. containing the species Serpulina hyodysenteriae comb. nov. and Serpulina innocens comb. nov. Int J Syst Bacteriol. 1992 Jan;42(1):189–190. doi: 10.1099/00207713-42-1-189. [DOI] [PubMed] [Google Scholar]
- Thiermann A. B., Handsaker A. L., Moseley S. L., Kingscote B. New method for classification of leptospiral isolates belonging to serogroup pomona by restriction endonuclease analysis: serovar kennewicki. J Clin Microbiol. 1985 Apr;21(4):585–587. doi: 10.1128/jcm.21.4.585-587.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. M., Arnett J. K., Heath J. D., Norris S. J. Treponema pallidum subsp. pallidum has a single, circular chromosome with a size of approximately 900 kilobase pairs. Infect Immun. 1991 Jul;59(7):2476–2479. doi: 10.1128/iai.59.7.2476-2479.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuerner R. L., Bolin C. A. Repetitive sequence element cloned from Leptospira interrogans serovar hardjo type hardjo-bovis provides a sensitive diagnostic probe for bovine leptospirosis. J Clin Microbiol. 1988 Dec;26(12):2495–2500. doi: 10.1128/jcm.26.12.2495-2500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuerner R. L., Herrmann J. L., Saint Girons I. Comparison of genetic maps for two Leptospira interrogans serovars provides evidence for two chromosomes and intraspecies heterogeneity. J Bacteriol. 1993 Sep;175(17):5445–5451. doi: 10.1128/jb.175.17.5445-5451.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuerner R. L. Physical map of chromosomal and plasmid DNA comprising the genome of Leptospira interrogans. Nucleic Acids Res. 1991 Sep 25;19(18):4857–4860. doi: 10.1093/nar/19.18.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Huurne A. A., van Houten M., Muir S., Kusters J. G., van der Zeijst B. A., Gaastra W. Inactivation of a Serpula (Treponema) hyodysenteriae hemolysin gene by homologous recombination: importance of this hemolysin in pathogenesis in mice. FEMS Microbiol Lett. 1992 Apr 1;71(1):109–113. doi: 10.1016/0378-1097(92)90550-8. [DOI] [PubMed] [Google Scholar]