Abstract
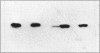
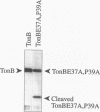
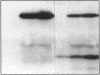
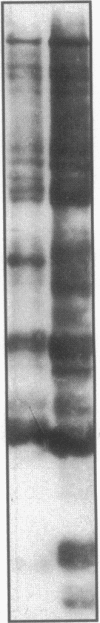
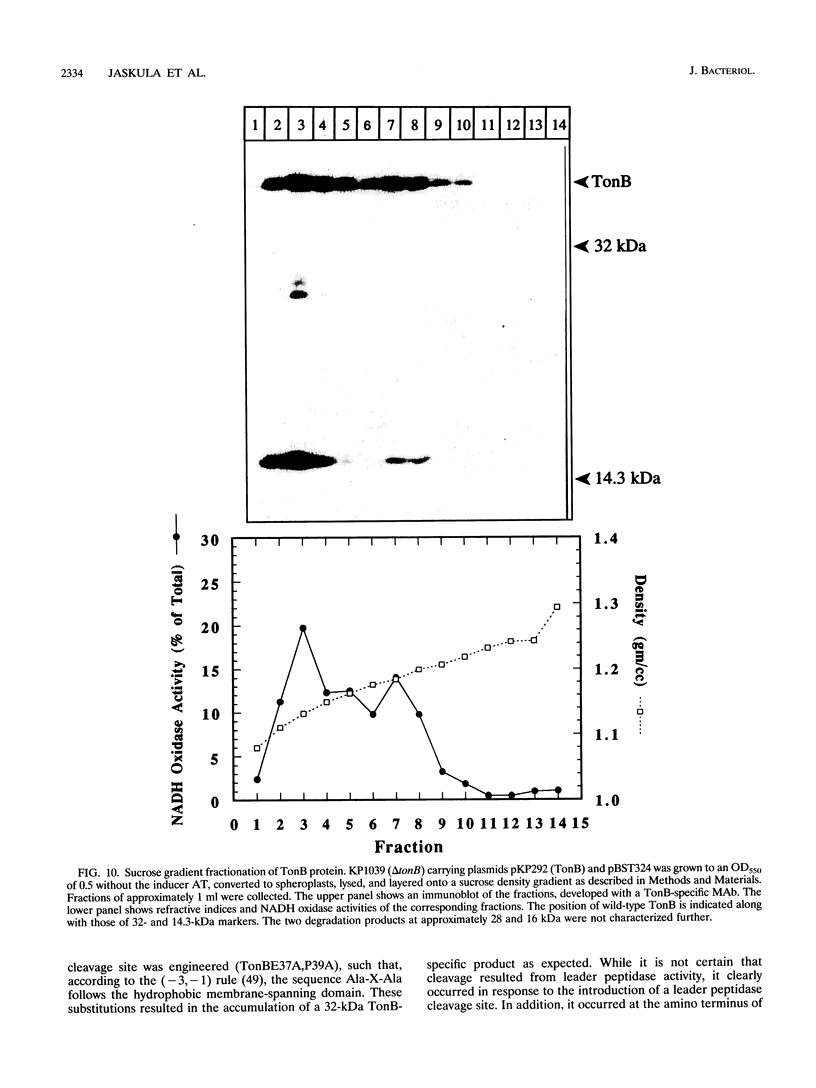
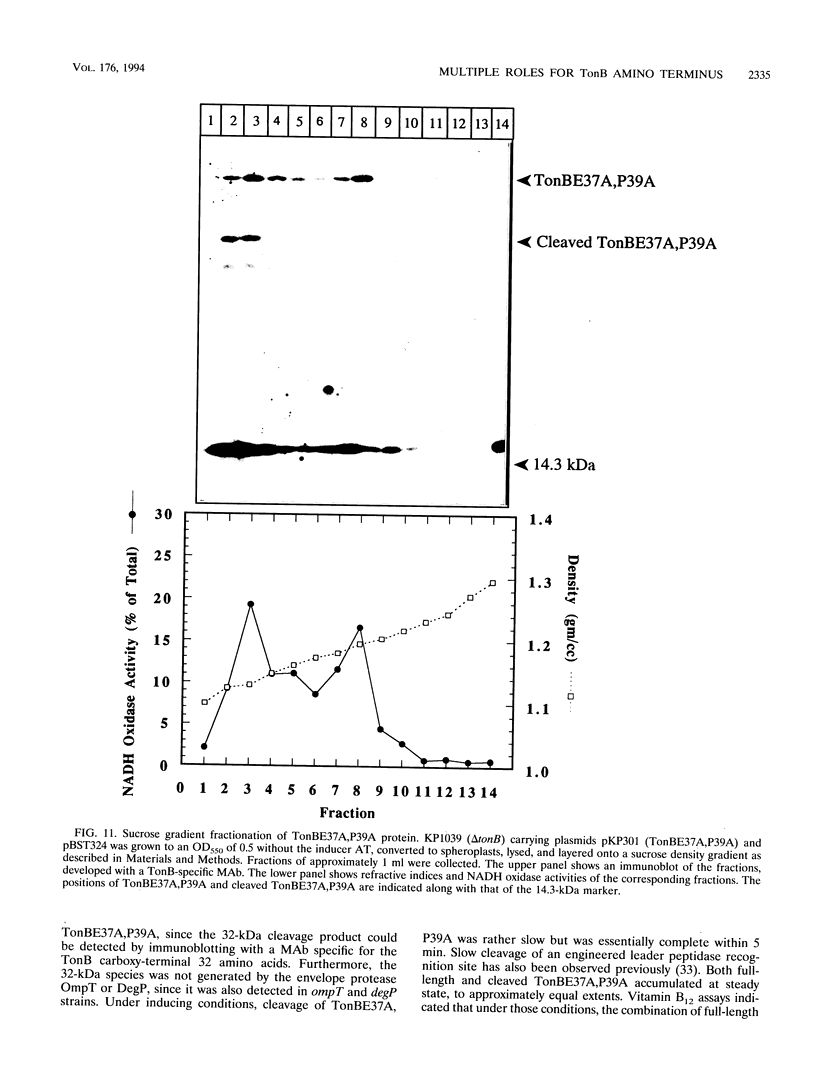
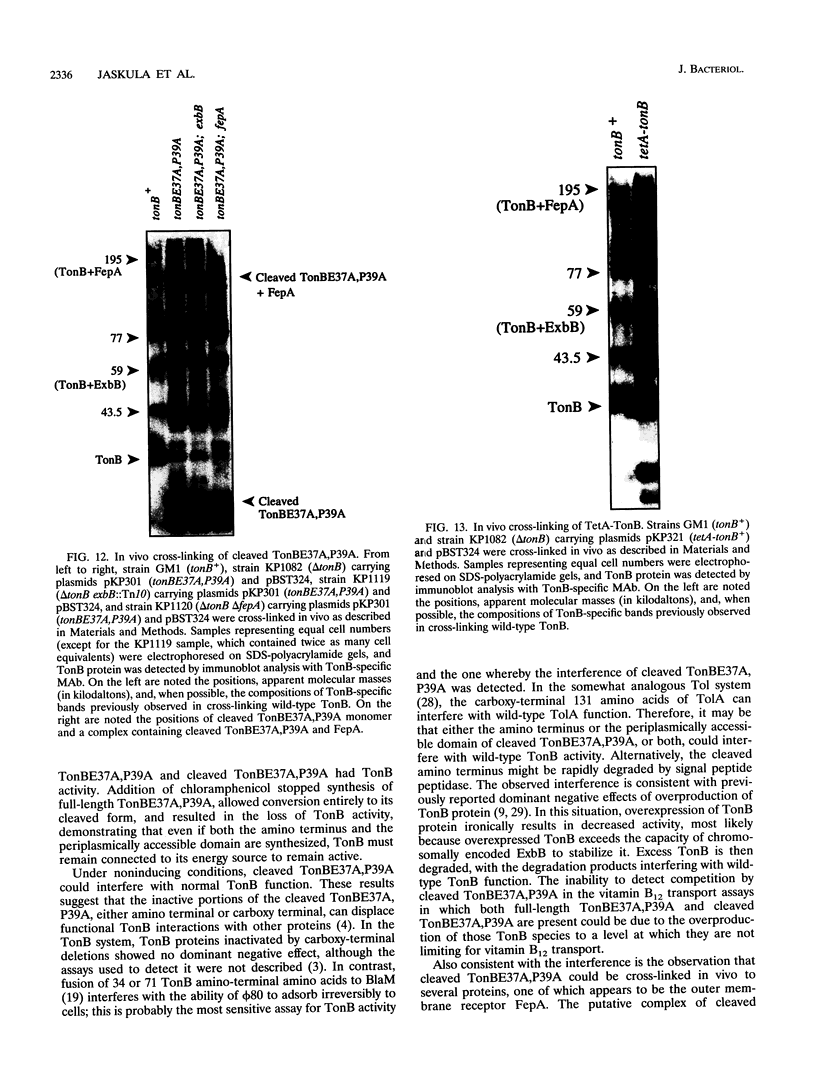
Escherichia coli TonB protein is an energy transducer, coupling cytoplasmic membrane energy to active transport of vitamin B12 and iron-siderophores across the outer membrane. TonB is anchored in the cytoplasmic membrane by its hydrophobic amino terminus, with the remainder occupying the periplasmic space. In this report we establish several functions for the hydrophobic amino terminus of TonB. A G-26-->D substitution in the amino terminus prevents export of TonB, suggesting that the amino terminus contains an export signal for proper localization of TonB within the cell envelope. Substitution of the first membrane-spanning domain of the cytoplasmic membrane protein TetA for the TonB amino terminus eliminates TonB activity without altering TonB export, suggesting that the amino terminus contains sequence-specific information. Detectable TonB cross-linking to ExbB is also prevented, suggesting that the two proteins interact primarily through their transmembrane domains. In vivo cleavage of the amino terminus of TonB carrying an engineered leader peptidase cleavage site eliminates (i) TonB activity, (ii) detectable interaction with a membrane fraction having a density intermediate to those of the cytoplasmic and outer membranes, and (iii) cross-linking to ExbB. In contrast, the amino terminus is not required for cross-linking to other proteins with which TonB can form complexes, including FepA. Additionally, although the amino terminus clearly is a membrane anchor, it is not the only means by which TonB associates with the cytoplasmic membrane. TonB lacking its amino-terminal membrane anchor still remains largely associated with the cytoplasmic membrane.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard J. D., Bertrand K. P. Membrane topology of the pBR322 tetracycline resistance protein. TetA-PhoA gene fusions and implications for the mechanism of TetA membrane insertion. J Biol Chem. 1992 Sep 5;267(25):17809–17819. [PubMed] [Google Scholar]
- Blair D. F., Kim D. Y., Berg H. C. Mutant MotB proteins in Escherichia coli. J Bacteriol. 1991 Jul;173(13):4049–4055. doi: 10.1128/jb.173.13.4049-4055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbeer C. The proton motive force drives the outer membrane transport of cobalamin in Escherichia coli. J Bacteriol. 1993 May;175(10):3146–3150. doi: 10.1128/jb.175.10.3146-3150.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Herrmann C. Evolutionary relationship of uptake systems for biopolymers in Escherichia coli: cross-complementation between the TonB-ExbB-ExbD and the TolA-TolQ-TolR proteins. Mol Microbiol. 1993 Apr;8(2):261–268. doi: 10.1111/j.1365-2958.1993.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Olson M. V. Oligodeoxynucleotide-directed mutagenesis of Escherichia coli and yeast by simple cotransformation of the primer and template. DNA. 1986 Aug;5(4):325–332. doi: 10.1089/dna.1986.5.325. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Barr G. C., Ni Bhriain N., Higgins C. F. DNA supercoiling and the anaerobic and growth phase regulation of tonB gene expression. J Bacteriol. 1988 Jun;170(6):2816–2826. doi: 10.1128/jb.170.6.2816-2826.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M. R., Riordan J. F. Membrane proteins with soluble counterparts: role of proteolysis in the release of transmembrane proteins. Biochemistry. 1991 Oct 22;30(42):10065–10074. doi: 10.1021/bi00106a001. [DOI] [PubMed] [Google Scholar]
- Fischer E., Günter K., Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989 Sep;171(9):5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günter K., Braun V. In vivo evidence for FhuA outer membrane receptor interaction with the TonB inner membrane protein of Escherichia coli. FEBS Lett. 1990 Nov 12;274(1-2):85–88. doi: 10.1016/0014-5793(90)81335-l. [DOI] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannavy K., Barr G. C., Dorman C. J., Adamson J., Mazengera L. R., Gallagher M. P., Evans J. S., Levine B. A., Trayer I. P., Higgins C. F. TonB protein of Salmonella typhimurium. A model for signal transduction between membranes. J Mol Biol. 1990 Dec 20;216(4):897–910. doi: 10.1016/S0022-2836(99)80009-6. [DOI] [PubMed] [Google Scholar]
- Hiles I. D., Powell L. M., Higgins C. F. Peptide transport in Salmonella typhimurium: molecular cloning and characterization of the oligopeptide permease genes. Mol Gen Genet. 1987 Jan;206(1):101–109. doi: 10.1007/BF00326543. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., McElhaney G. Outer membrane-dependent transport systems in Escherichia coli: turnover of TonB function. J Bacteriol. 1978 Jun;134(3):1020–1029. doi: 10.1128/jb.134.3.1020-1029.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfenkel K., Braun V. Membrane topology of the Escherichia coli ExbD protein. J Bacteriol. 1992 Aug;174(16):5485–5487. doi: 10.1128/jb.174.16.5485-5487.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfenkel K., Braun V. Topology of the ExbB protein in the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1993 Mar 15;268(8):6050–6057. [PubMed] [Google Scholar]
- Karlsson M., Hannavy K., Higgins C. F. A sequence-specific function for the N-terminal signal-like sequence of the TonB protein. Mol Microbiol. 1993 Apr;8(2):379–388. doi: 10.1111/j.1365-2958.1993.tb01581.x. [DOI] [PubMed] [Google Scholar]
- Karlsson M., Hannavy K., Higgins C. F. ExbB acts as a chaperone-like protein to stabilize TonB in the cytoplasm. Mol Microbiol. 1993 Apr;8(2):389–396. doi: 10.1111/j.1365-2958.1993.tb01582.x. [DOI] [PubMed] [Google Scholar]
- Klebba P. E., Rutz J. M., Liu J., Murphy C. K. Mechanisms of TonB-catalyzed iron transport through the enteric bacterial cell envelope. J Bioenerg Biomembr. 1993 Dec;25(6):603–611. doi: 10.1007/BF00770247. [DOI] [PubMed] [Google Scholar]
- Koebnik R. The molecular interaction between components of the TonB-ExbBD-dependent and of the TolQRA-dependent bacterial uptake systems. Mol Microbiol. 1993 Jul;9(1):219–219. doi: 10.1111/j.1365-2958.1993.tb01683.x. [DOI] [PubMed] [Google Scholar]
- Kuo D. W., Chan H. K., Wilson C. J., Griffin P. R., Williams H., Knight W. B. Escherichia coli leader peptidase: production of an active form lacking a requirement for detergent and development of peptide substrates. Arch Biochem Biophys. 1993 Jun;303(2):274–280. doi: 10.1006/abbi.1993.1283. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen R. A., Wood G. E., Postle K. The conserved proline-rich motif is not essential for energy transduction by Escherichia coli TonB protein. Mol Microbiol. 1993 Dec;10(5):943–953. doi: 10.1111/j.1365-2958.1993.tb00966.x. [DOI] [PubMed] [Google Scholar]
- Levengood-Freyermuth S. K., Click E. M., Webster R. E. Role of the carboxyl-terminal domain of TolA in protein import and integrity of the outer membrane. J Bacteriol. 1993 Jan;175(1):222–228. doi: 10.1128/jb.175.1.222-228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUSHIRO A. Specialized transduction of tryptophan markers in Escherichia coli K12 by bacteriophage phi-80. Virology. 1963 Apr;19:475–482. doi: 10.1016/0042-6822(63)90041-2. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Saier M. H., Jr Transport proteins in bacteria: common themes in their design. Science. 1992 Nov 6;258(5084):936–942. doi: 10.1126/science.1279804. [DOI] [PubMed] [Google Scholar]
- Nilsson I., von Heijne G. A de novo designed signal peptide cleavage cassette functions in vivo. J Biol Chem. 1991 Feb 25;266(6):3408–3410. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pakula A. A., Simon M. I. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Good R. F. DNA sequence of the Escherichia coli tonB gene. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5235–5239. doi: 10.1073/pnas.80.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Skare J. T. Escherichia coli TonB protein is exported from the cytoplasm without proteolytic cleavage of its amino terminus. J Biol Chem. 1988 Aug 5;263(22):11000–11007. [PubMed] [Google Scholar]
- Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990 Dec;4(12):2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993 Dec;25(6):591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- Reynolds P. R., Mottur G. P., Bradbeer C. Transport of vitamin B12 in Escherichia coli. Some observations on the roles of the gene products of BtuC and TonB. J Biol Chem. 1980 May 10;255(9):4313–4319. [PubMed] [Google Scholar]
- Roof S. K., Allard J. D., Bertrand K. P., Postle K. Analysis of Escherichia coli TonB membrane topology by use of PhoA fusions. J Bacteriol. 1991 Sep;173(17):5554–5557. doi: 10.1128/jb.173.17.5554-5557.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Kidd S., Kelley M. R. An improved filamentous helper phage for generating single-stranded plasmid DNA. Gene. 1986;45(3):333–338. doi: 10.1016/0378-1119(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Skare J. T., Ahmer B. M., Seachord C. L., Darveau R. P., Postle K. Energy transduction between membranes. TonB, a cytoplasmic membrane protein, can be chemically cross-linked in vivo to the outer membrane receptor FepA. J Biol Chem. 1993 Aug 5;268(22):16302–16308. [PubMed] [Google Scholar]
- Skare J. T., Postle K. Evidence for a TonB-dependent energy transduction complex in Escherichia coli. Mol Microbiol. 1991 Dec;5(12):2883–2890. doi: 10.1111/j.1365-2958.1991.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Skare J. T., Roof S. K., Postle K. A mutation in the amino terminus of a hybrid TrpC-TonB protein relieves overproduction lethality and results in cytoplasmic accumulation. J Bacteriol. 1989 Aug;171(8):4442–4447. doi: 10.1128/jb.171.8.4442-4447.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Sun T. P., Webster R. E. fii, a bacterial locus required for filamentous phage infection and its relation to colicin-tolerant tolA and tolB. J Bacteriol. 1986 Jan;165(1):107–115. doi: 10.1128/jb.165.1.107-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Christie G. E., Platt T. Tandem termination sites in the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1981 May;78(5):2913–2917. doi: 10.1073/pnas.78.5.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. The signal peptide. J Membr Biol. 1990 May;115(3):195–201. doi: 10.1007/BF01868635. [DOI] [PubMed] [Google Scholar]