Abstract
Cases of familial amyotrophic lateral sclerosis (fALS; a neurodegenerative disorder) have been reported in which the gene for Cu/Zn superoxide dismutase (CuZnSOD) was mutated. Several studies with the fALS mutant CuZnSOD in transgenic mice and cells showed that the fALS mutations act through an as yet undefined dominant gain-of-function mechanism. Wild-type CuZnSOD catalyzes the dismutation of superoxide (O2⨪) but also produces hydroxyl radicals (•OH) with H2O2 as substrate. Two laboratories have recently demonstrated that the •OH production ability was preferentially enhanced by the fALS mutant CuZnSOD, suggesting that this might be the function gained in fALS. In this study, we used transgenic CuZnSOD (Tg-CuZnSOD) mice with elevated levels of CuZnSOD to determine whether overexpression of wild-type CuZnSOD was also associated with increased •OH production and impaired muscle function. Enhanced formation of •OH was detected, by spin trapping, in brain and muscle extracts of the Tg-CuZnSOD mice. Three independently derived Tg-CuZnSOD lines showed muscle abnormalities, reflected by altered electromyography (EMG) and diminished performance in the rope grip test. After treatment with paraquat (PQ), a widely used herbicide and O2⨪-generating compound, muscle disability significantly deteriorated in Tg-CuZnSOD mice but not in control mice. The results indicate that elevated levels of CuZnSOD cause indigenous long-term oxidative stress leading to impairment of muscle function. These findings may provide valuable clues about the concurred role of indigenous oxidative stress and exogenous agents in the etiology of sporadic ALS and several other neurodegenerative diseases in which a specific subset of neurons is affected.
The enzyme Cu/Zn superoxide dismutase (CuZnSOD), which catalyzes the conversion of superoxide radicals (O2⨪) into H2O2, plays an important role in the metabolism of oxygen free radicals (1). Although numerous studies have implicated oxygen free radicals in a broad range of neuropathologies (reviewed in refs. 2–4), the recent discovery that mutations in the CuZnSOD gene are responsible for cases of familial amyotrophic lateral sclerosis (fALS) (5, 6) provided clear genetic evidence that altered metabolism of oxygen free radicals could be involved in neurodegenerative diseases (reviewed in refs. 7 and 8). We (9–14) and others (15–22) have shown that a long-term increase in CuZnSOD or MnSOD (23, 24) activity causes oxidative injury-mediated phenotypic changes. Using model systems of transgenic cells and mice, we found that stably transfected cells overexpressing CuZnSOD showed substantially increased lipid peroxidation associated with a specific lesion affecting the chromaffin granule’s proton pump (11, 12). This proton pump plays an important role in the uptake of neurotransmitters. A similar defect was also identified in the platelet’s dense granules of the transgenic CuZnSOD (Tg-CuZnSOD) mice, which are the organelle responsible for the uptake and storage of blood serotonin (13). In addition, Tg-CuZnSOD mice have certain oxidative stress-induced lesions in the thymus and bone marrow (25), and cultured Tg-CuZnSOD neurons exhibit higher susceptibility to kainic acid-induced apoptosis (26). These findings indicated that oxidative damage caused by increased CuZnSOD can have a highly specific subcellular target and may serve as a paradigm for how alterations in CuZnSOD activity could cause oxidative stress-mediated cell injury leading to a neurodegenerative disease like ALS (7, 8). Of note, the neuromuscular junctions of Tg-CuZnSOD mice display significant abnormalities and morphological changes (9, 10, 14). These defects, although subclinical, are reminiscent of the pathology observed in transgenic mice with ALS-like disease (27) that bear a mutant CuZnSOD transgene with the fALS mutations (28–30). Although initially it was suggested that the fALS mutations cause a reduction in enzyme activity (31–34), the transgenic fALS mutant CuZnSOD mice (27–30) and cells (35, 36) clearly showed that ALS mutations act through a dominant gain-of-function that has yet to be identified.
Besides dismutation (O2⨪ + O2⨪ → H2O2), CuZnSOD catalyzes surrogate reactions, such as the production of hydroxyl radicals (•OH) using anionic scavengers and H2O2 (37, 38). Hydroxyl radicals are extremely reactive and noxious oxygen free radical species. Upon formation, a hydroxyl radical will immediately react with almost any biological macromolecule, causing oxidative damage (2–4). Recently, two reports (39, 40) have demonstrated that of the two reactions catalyzed by native CuZnSOD, i.e., dismutation (1) and production of •OH (37, 38), the latter is significantly enhanced by the fALS mutant CuZnSOD (39, 40), which suggests that this might be the function gained in fALS.
Under normal conditions, most of the H2O2 molecules generated by CuZnSOD are further metabolized to water by glutathione peroxidase (1–4). However, when the activity of CuZnSOD is increased, as in Tg-CuZnSOD mice, without a concomitant increase in glutathione peroxidase, H2O2 accumulates (12, 19, 22, 25, 26), and the CuZnSOD-catalyzed production of •OH could be facilitated. We used Tg-CuZnSOD mice overexpressing CuZnSOD to investigate whether elevated activity of wild-type CuZnSOD causes enhanced production of •OH and whether this phenomenon affects muscle function.
MATERIALS AND METHODS
Tg-CuZnSOD Mice.
Transgenic mice harboring the human CuZnSOD gene were obtained as described (9, 41) by microinjecting fertilized eggs with a linear 14.5-kb fragment of human genomic DNA containing the entire CuZnSOD gene, including its regulatory sequences. Tg-CuZnSOD male and female mice homozygous for the transgene were bred and used for the present experiments. The mice were kept under pathogen-free conditions. All experiments were carried out with male Tg-CuZnSOD and age-matched control mice. The three strains studied here, Tg-51, Tg-69, and Tg-70, contained four to five copies of the human CuZnSOD gene in their genomes and expressed the transgene as an active enzyme (9, 25, 26). To measure the specific activity of CuZnSOD in muscle and brain, extracts were prepared in 0.5% Nonidet P-40 and assayed by the spectrophotometric method of inhibition of nitrite formation from hydroxylammonium chloride (9). The specific activity of CuZnSOD in cell extracts was calculated according to a standard curve using homogenous human CuZnSOD (Biotechnology General, Rehovot, Israel). Whereas CuZnSOD activity was elevated in tissues of Tg-CuZnSOD relative to control mice (9, 25, 26, 41), the activities of both glutathione peroxidase (42) and MnSOD (43) in organs of the transgenic mice were remarkably similar to those of control mice. PQ, a well known O2⨪-generating compound (44), was added to the drinking water. Based on their water consumption, mice obtained 10–20 mg of PQ per kilogram of body weight per day for 18 months.
Electron Paramagnetic Resonance (EPR) Spectroscopy.
Extracts were assayed as described (37–40) with minor modifications. Equal amounts (50 μl) of muscle and brain cell-free extracts (in PBS) from Tg-CuZnSOD and control mice were preadjusted to contain equal amounts of protein. The extracts were mixed with 50 μl of PBS containing 30 mM H2O2 and 100 mM activated charcoal-purified 5,5-dimethyl-1-pyrroline N-oxide (DMPO). The mixture was immediately pipetted into a 100-μl flat cell, and EPR spectra were instantly acquired at room temperature using an ER 200 D-SRC spectrometer (Bruker, Germany). The spectrometer settings were as follows: receiver gain, 1.25 × 106; modulation amplitude, 1 G; time constant, 1.25 sec; sweep time, 500 sec; center field, 3,500 G; sweep width, 100 G; microwave power, 20 mW.
The metal chelator RL252 was synthesized by Avi Shanzer (45). Either RL252 (100 μM) or diethyldithiocarbamate (100 μM) were used with 30 mM H2O2 and 3 μM wild-type CuZnSOD.
EMG.
Groups of coded mice (n = 4–6), either control or Tg-CuZnSOD (lines Tg-51, Tg-69, and Tg-70), treated and untreated with PQ were anesthetized with sodium pentothal (1.2–1.8 mg per mouse i.p.), and needle EMG was performed with a bipolar EMG needle electrode inserted in multiple sites into the gastrocnemius and interosseous muscles of one hind limb. Recording was done with a conventional EMG apparatus (Medelec, Old Woking, Surrey, U.K.). Studies were performed with coded mice so that the electromyographer was blinded as to which mice were being tested. For each mouse, EMG findings were graded on a 0–3 scale designated the “EMG Pathology Index” as follows: 0, no spontaneous activity and normal insertional activity; 1, sparse fibrillation potentials or positive sharp waves occurring only in one or two sites within the muscles; 2, fibrillations in more than 50% of muscle sites and/or markedly increased insertional activity in most sites; 3, abundant fibrillations in most sites and prolonged insertional activity.
Hind Paw Footprint Ink Test.
Hind leg paralysis in mice was followed by measuring the length of their stride as described by Gurney (28). Briefely, the soles of the hind legs were dipped in ink, and the mice were made to walk inside a long narrow track lined with paper. The resultant ink footprints were analyzed, and the average length of three strides for each leg was determined. Control and two Tg-CuZnSOD lines (Tg-51 and Tg-69) were examined.
Rope Grip Test.
The assay was conducted as described by Hall et al. (46) with some modifications. Four-month-old mice were allowed to grip with their front legs a 2-mm thick horizontal tight rope placed 80 cm above the ground (see Fig. 3), and the time elapsed until the mice raised their hind legs to grip the rope was measured. If the mouse gripped the rope by both hind legs within 60 sec and kept the grip for at least 10 sec, the mouse was recorded as a success. Each group contained 5–10 mice, all of which were analyzed twice on two different days, each time in three repetitions with ≈30-min intervals. Treatment with PQ at 40 mg per kg per day was carried out over 3 months. Statistical analysis included the actual time recorded until a successful grip occurred. Mice that failed to grip with their hind legs were scored as 60 sec. P values were determined by Student’s t test.
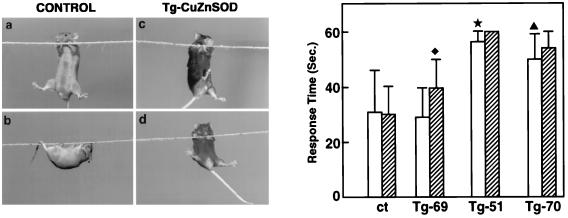
Figure 3.
Sensorimotor performance of Tg-CuZnSOD and control mice. (Left) The rope grip test was conducted as described in Material and Methods. Four-month-old mice were allowed to grip with their front legs a 2-mm-thick horizontal tight rope placed 80 cm above the ground (a and c), and the time elapsed until the mice raised their hind legs to grip the rope (b and d) was measured. If the mouse gripped the rope by both hind legs within 60 sec and kept the grip for at least 10 sec, the mouse was recorded as a success. (Right) Each group contained 5–10 mice, all of which were analyzed twice on two different days, each time with three repetitions. Numbers indicate the average of total repetitions. Results are expressed as the mean ± SEM. Four-month-old Tg-CuZnSOD mice (open bars) and PQ-treated (hatched bars) differed significantly from control mice (★, P = 0.01; ♦, P = 0.03; ▴, P = 0.05). P values were determined by Student’s t test.
RESULTS AND DISCUSSION
•OH Are Generated in Tissue Extracts of Tg-CuZnSOD.
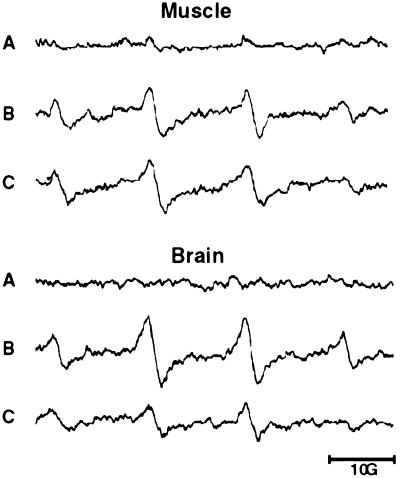
CuZnSOD catalyzed production of •OH in muscle and brain extracts of Tg-CuZnSOD and nontransgenic control mice was measured by the previously used spin-trap method (37–40). In the presence of H2O2 and the spin trap, DMPO, CuZnSOD catalyzed the formation of the hydroxyl adduct DMPO-OH, which can be detected by EPR spectroscopy (Fig. 1). DMPO-OH spin adduct appears as four evenly spaced signals with intensity ratios of 1:2:2:1 and aN = aβH = 14.9 G (47). The peak intensity of the signals is proportional to the amount of •OH formed in the sample. Muscle and brain extracts of two Tg-CuZnSOD mouse lines (Tg-51 and Tg-69) produced significantly higher amounts of DMPO-OH than extracts of control nontransgenic mice (Fig. 1). Heat inactivation or the addition of metal chelators such as diethyldithiocarbamate or RL252 (45) abolished the signal. Two points strongly suggest that •OH formation in Tg-CuZnSOD tissue extracts represents the in vivo situation. The height of the EPR signals in Fig. 1, which was attained at about 5 min, did not significantly change during the following 20 min of incubation. This result indicated that in the extracts the enzyme was relatively protected against inactivation by H2O2. Second, increase in the in vivo production of H2O2, a key element in the CuZnSOD-mediated •OH generation, was previously detected in cells of Tg-CuZnSOD mice (25, 26).
Figure 1.
Formation of •OH in tissue extracts of Tg-CuZnSOD and control mice. EPR spectra of DMPO-OH adducts formed in extracts of muscle (upper traces) and brain (lower traces) were assayed as described. (A) Control. (B) Tg-51. (C) Tg-69. The spectra shown are representative and were recorded about 5 min after mixing the reagents; measurements were repeated several times using freshly prepared extracts from different animals. EPR spectra were acquired at room temperature, using an ER 200 D-SRC spectrometer (Bruker, Germany). The EPR spectra depict the typical four DMPO-OH signals and indicate that more •OH are produced by extracts from Tg-CuZnSOD mice.
Tg-CuZnSOD Mice Exhibit EMG Abnormalities.
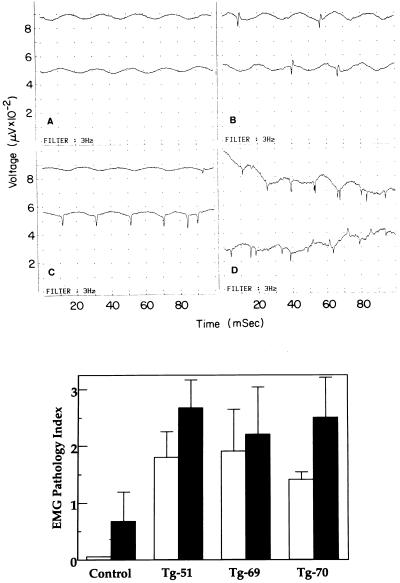
Under regular maintenance conditions, Tg-CuZnSOD mice showed no obvious physical abnormalities even though they had certain histopathological defects in their neuromuscular junctions (9, 10, 14) and motor axons (27). Deterioration in muscle function is often preceded by electrophysiological changes that can be detected by EMG. Changes in EMG provide a sensitive and timely predictive marker for early muscular degeneration/denervation. Direct EMG recording at multiple sites of the hind limb muscle revealed the presence of pathological changes in Tg-CuZnSOD mice (Fig. 2), including spontaneous activity (fibrillation potentials and positive sharp waves) as well as markedly prolonged insertional activity (“pseudomyotonic” discharges). Young (4-month-old) Tg-CuZnSOD mice (from the three transgenic lines, Tg-51, Tg-69, and Tg-70) had a significantly higher score of EMG Pathology Index, compared with control mice (P < 0.05; Fig. 2). The EMG changes were more pronounced in older (≈2 years) Tg-CuZnSOD mice (data not shown) and worsened (P < 0.005) by treatment with PQ, a superoxide radicals-generating compound (Fig. 2). Thus, EMG analysis of the hind limb muscles demonstrated that Tg-CuZnSOD mice from the three different lines had pronounced pseudomyotonic activity and fibrillations that are usually associated with muscle denervation. Significantly, PQ preferentially exacerbated the deterioration of muscle electrical activity in the Tg-CuZnSOD mice, further emphasizing the role of CuZnSOD and H2O2-mediated oxidative stress in this process.
Figure 2.
EMG analysis of transgenic and control mice. (Upper) EMG abnormalities in the hind limb muscle of Tg-CuZnSOD mice. EMG recording from the hind limb muscle of Tg-CuZnSOD and control mice. The signals are representative and show no electrical activity at rest (“normal”; A), fibrillation potentials at rest (B), positive sharp waves at rest (C), and a train of insertional positive waves (“pseudomyotonic discharge”; D). Measurements were conducted on groups of coded mice (n = 4–6), either control or Tg-CuZnSOD (lines Tg-51, Tg-69, and Tg-70), treated and untreated with PQ. Tested muscles were evaluated for the presence of spontaneous activity (fibrillation potentials and positive sharp waves; B and C) and for markedly prolonged insertional activity (“pseudomyotonic” discharges or long trains of insertional positive waves lasting more than 2 sec; D). (Lower) For each mouse, EMG findings were graded on a 0–3 scale designated the EMG Pathology Index. All EMG studies were performed with coded mice so that the electromyographer was blinded as to which mice were being tested. Results are expressed as the mean ± SEM. Four-month-old Tg-CuZnSOD mice (open bars) differed significantly from control mice (P < 0.05) as did 2-year-old PQ-treated Tg-CuZnSOD mice (solid bars; P < 0.005). P values were determined by Student’s t test.
Muscle Function Is Impaired in Tg-CuZnSOD Mice.
Despite the apparent abnormal EMG recording and the defects in neuromuscular junctions (9, 10) and motor axons (27), the Tg-CuZnSOD mice walked normally and did not display the signs of paralytic muscle dysfunction seen in mice overexpressing the fALS mutant CuZnSOD (28–30). To substantiate these observations, the stride lengths of transgenic and control mice were measured using the hind paw footprint ink test described by Gurney et al. (28). Each group contained four to six mice, and the average length of stride was calculated. The stride length of 4-month-old control mice was 6.8 ± 0.7 cm (mean ± SEM) and did not significantly differ in Tg-51 and Tg-69 mice even when mice were treated with PQ. In transgenic fALS mutant CuZnSOD mice, a worsening of symptoms with age was reported (28–30). We therefore subjected older mice (≈2 years) to the footprint ink test. A small but significant shortening of the stride length in 2-year-old PQ-treated Tg-51 mice was observed: from 6.7 ± 0.4 cm (n = 6) to 6.1 ± 0.3 cm (n = 4; P = 0.005). Clearly, deterioration in Tg-CuZnSOD muscle function was not severe enough to register in the footprint ink test previously used to monitor ALS-like paralysis in the fALS mutant CuZnSOD transgenic mice (28).
To further delineate the defects in muscle function, Tg-CuZnSOD and control mice were subjected to the rope grip test. This test evaluates the sensorimotor-coordinated activity of limb, abdomen, and back muscles that enables mice to grip the rope (Fig. 3). The results indicated a significant difference between control and Tg-CuZnSOD mice. Control mice usually gripped the rope with their two hind legs within 20–30 sec and walked backwards along it while hanging upside down (Fig. 3). In contrast, most Tg-CuZnSOD mice (Tg-51 and Tg-70) failed to grip the rope with their hind legs during the first 50 sec, and many did not grip it until the 60-sec end point of the test (Fig. 3). In accord with the EMG data, PQ treatment caused further deterioration in the grip test performance of Tg-CuZnSOD mice; more mice failed the test within the 60-sec end point, whereas the performance of control mice was not affected (Fig. 3). Tg-69 mice performed similarly to control mice except when treated with PQ (Fig. 3). This was unexpected since Tg-69 mice had pathological changes in their neuromuscular junctions (9, 10, 14), as well as an altered EMG pattern (Fig. 2). The CuZnSOD activity in various organs of Tg-69 mice was always higher than that in control mice (see Materials and Methods and refs. 25 and 26), as evidenced by the •OH production of their muscle and brain extracts (Fig. 1). As mentioned above, the grip-test assesses diverse sensorimotor functions that require a coordinative action of multiple neuromuscular elements in which genetic background of the animal may play a role. Similar phenomena were previously observed by other investigators (48). Thus, although Tg-69 mice exhibited oxidative stress-mediated changes in their muscles, manifested by abnormal EMG, the more complex grip test performance was apparently affected by their genetic background. Consistent with this, although treatment with PQ did not affect control mice, it significantly influenced the grip test performance of Tg-69 mice (Fig. 3). Transgenic mice, in general, have mixed genetic backgrounds; thus, genetic heterogeneity exists even within a restricted lineage. We have therefore used throughout this work, three Tg-CuZnSOD lines in which the transgene has been integrated into different chromosomal sites.
Taken together, the results of the EMG recordings and grip test assay show a clear deterioration in the muscle performance of Tg-CuZnSOD mice compared with nontransgenic mice. The enhanced •OH production in tissue extracts and the preferential effect of PQ strongly indicate the involvement of oxygen radicals in the impaired muscle function of Tg-CuZnSOD mice. As noted above, PQ causes increased production of superoxide radicals, the vast majority of which are rapidly converted by CuZnSOD to H2O2. Thus, in Tg-CuZnSOD mice, treatment with PQ increased the levels of the two key elements in •OH production, CuZnSOD and H2O2, thereby facilitating the reaction shown in Fig. 1 and exacerbating muscle deterioration. However, even without PQ, the level of H2O2 production in Tg-CuZnSOD mice is higher than in control mice (25, 26), and •OH production could therefore be enhanced.
The results show that long-term overexpression of CuZnSOD, even of the wild-type enzyme, causes oxidative stress-mediated impairment in muscle function that is specifically exacerbated by treatment with PQ. Our previous work has demonstrated that Tg-CuZnSOD neurons are more susceptible to kainic acid-mediated excitotoxicity, reflected by an earlier onset and enhanced apoptotic cell death (26). Together these data may explain the role indigenous oxidative stress and exogenous agents play in sporadic ALS and several other neurodegenerative diseases in which a specific subset of neurons are affected (3, 4, 7, 8). The etiology of these disorders is unknown but is thought to involve common mechanisms of programmed cell death that are induced by a variety of risk factors, including genetic and environmental factors (2–4).
Acknowledgments
We thank Yehudit Hermesh for skillful technical assistance, A. Shanzer for providing the RL252, and H. Soreq and M. Rubinstein for valuable discussions. This work was supported by grants from the National Institutes of Health (HD21229); the Fritz Thyssen Stiftung (Germany); Mr. Bernard Sabrier, Geneva; the Weizmann Institute’s Forchheimer Center of Molecular Genetics; and the Shapell Family Biomedical Research Foundation at the Weizmann Institute.
ABBREVIATIONS
- CuZnSOD
Cu/Zn superoxide dismutase
- Tg-CuZnSOD
transgenic CuZnSOD
- fALS
familial amyotrophic lateral sclerosis
- EPR
electron paramagnetic resonance
- DMPO
5,5-dimethyl-1-pyrroline N-oxide
- EMG
electromyography
- PQ
paraquat
References
- 1.Fridovich I. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 2.Olanow C W. Trends Neurosci. 1993;16:439–444. doi: 10.1016/0166-2236(93)90070-3. [DOI] [PubMed] [Google Scholar]
- 3.Ames B M, Shigenaga M K, Hagen T. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beal M F. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 5.Rosen D R, Siddique T, Patterson D, Figlewicz D A, Sapp P, et al. Nature (London) 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 6.Deng H X, Hentati A, Tainer J A, Iqbal Z, Cayabyab A, Hung W-Y, Getzoff E D, Hu P, Herzfeldt B, Roos R P, Warner C, Deng G, Soriano E, Smith C, Parge H E, Ahmed A, Roses A D, Hallewell R A, Pericak-Vance M A, Siddique T. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 7.Brown R H. Cell. 1995;80:687–692. doi: 10.1016/0092-8674(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 8.Smith R G, Appel S H. Annu Rev Med. 1995;46:133–145. doi: 10.1146/annurev.med.46.1.133. [DOI] [PubMed] [Google Scholar]
- 9.Avraham K B, Schickler M, Sapoznikov D, Yarom R, Groner Y. Cell. 1988;54:823–829. doi: 10.1016/s0092-8674(88)91153-1. [DOI] [PubMed] [Google Scholar]
- 10.Avraham K B, Sugarman H, Rotshenker S, Groner Y. J Neurocytol. 1991;20:208–215. doi: 10.1007/BF01186993. [DOI] [PubMed] [Google Scholar]
- 11.Elroy-Stein O, Bernstein Y, Groner Y. EMBO J. 1986;5:615–622. doi: 10.1002/j.1460-2075.1986.tb04255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elroy-Stein O, Groner Y. Cell. 1988;52:259–267. doi: 10.1016/0092-8674(88)90515-6. [DOI] [PubMed] [Google Scholar]
- 13.Schickler M, Knobler H, Avraham K B, Elroy-Stein O, Groner Y. EMBO J. 1989;8:1385–1392. doi: 10.1002/j.1460-2075.1989.tb03519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarom R, Sapoznikov D, Havivi Y, Avraham K B, Schickler M, Groner Y. J Neurol Sci. 1988;88:41–53. doi: 10.1016/0022-510x(88)90204-3. [DOI] [PubMed] [Google Scholar]
- 15.Norris H K, Hornsby P J. Mutat Res. 1990;237:95–106. doi: 10.1016/0921-8734(90)90015-j. [DOI] [PubMed] [Google Scholar]
- 16.Amstad P, Peskin A, Shah G, Mirault M-E, Moret R, Zibinden I, Cerutti P. Biochemistry. 1991;30:9305–9313. doi: 10.1021/bi00102a024. [DOI] [PubMed] [Google Scholar]
- 17.Nelson S K, Bose S K, McCord J M. Free Radical Biol Med. 1994;16:195–200. doi: 10.1016/0891-5849(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 18.McCord J M. Science. 1994;226:1586–1587. [PubMed] [Google Scholar]
- 19.Kelner M J, Bagnell R, Montoya M, Estes L, Uglick S F, Cerutti P. Free Radical Biol Med. 1995;18:497–506. doi: 10.1016/0891-5849(94)00167-i. [DOI] [PubMed] [Google Scholar]
- 20.Ditelberg, J. S., Sheldon, R. A., Epstein, C. J. & Ferriero, D. M. (1995) Neurobiology 45, Suppl., A391 (abstr.).
- 21.De Vos S, Epstein C J, Carlson E, Cho S K, Koeffler H P. Biochem Biophys Res Commun. 1995;208:523–531. doi: 10.1006/bbrc.1995.1370. [DOI] [PubMed] [Google Scholar]
- 22.De Haan J B, Cristiano F, Iannello R, Kelner M J, Kola I. Hum Mol Genet. 1996;5:283–292. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]
- 23.Li J-J, Oberley L W, St Clair D K, Ridnour L A, Oberley T D. Oncogene. 1995;10:1989–2000. [PubMed] [Google Scholar]
- 24.Zhong W, Oberley L W, Oberley T D, Yan T, Domann F E, St Clair D K. Cell Growth Differ. 1996;7:1175–1186. [PubMed] [Google Scholar]
- 25.Peled-Kamar M, Lotem J, Okon E, Sachs L, Groner Y. EMBO J. 1995;14:4985–4993. doi: 10.1002/j.1460-2075.1995.tb00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar-Peled O, Korkotian E, Segal M, Groner Y. Proc Natl Acad Sci USA. 1996;93:8530–8535. doi: 10.1073/pnas.93.16.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dal Canto M C, Gurney M E. Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- 28.Gurney M E, Pu H, Chiu A Y. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 29.Ripps M E, Huntley G W, Hof P R, Morrison J M, Gordon J W. Proc Natl Acad Sci USA. 1995;92:689–693. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong C P, Pardo C A, Borchelt D R, Lee M K, Copeland N G, Jenkins N A, Sisodia S S, Cleveland D W, Price D L. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 31.Bowling A C, Schulz J B, Brown R H, Beal M F. J Neurochem. 1993;61:2322–2325. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 32.Robberecht W, Sapp P, Viaene M K, Rosen D, McKenna-Yasek D, Haines J, Horvitz R, Theys P, Brown R J., Jr J Neurochem. 1994;62:384–387. doi: 10.1046/j.1471-4159.1994.62010384.x. [DOI] [PubMed] [Google Scholar]
- 33.Rosen D R, Bowling A C, Patterson D, Usdin T B, Sapp P, Mezey E, McKenna-Yasek D, O’Regan J P, Rahmani Z, Ferrante R J, Brownstein M J, Kowall N W, Beal M F, Horvitz H R, Brown R H., Jr Hum Mol Genet. 1994;3:981–987. doi: 10.1093/hmg/3.6.981. [DOI] [PubMed] [Google Scholar]
- 34.Orrell R, deBelleroche J, Marklund S, Bowe F, Hallewell R. Nature (London) 1995;374:504–505. doi: 10.1038/374504a0. [DOI] [PubMed] [Google Scholar]
- 35.Rabizadeh S, Gralla E B, Borchelt D R, Gwinn R, Valentine J S, Sisodia S, Wong P, Lee M, Hahn H, Bredesen D E. Proc Natl Acad Sci USA. 1995;92:3024–3028. doi: 10.1073/pnas.92.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borchelt D R, Guarnieri M, Wong P C, Lee M K, Slunt H S, Xu Z S, Sisodia S S, Price D L, Cleveland D W. J Biol Chem. 1995;270:3234–3238. doi: 10.1074/jbc.270.7.3234. [DOI] [PubMed] [Google Scholar]
- 37.Yim M B, Chock P B, Stadtman E R. Proc Natl Acad Sci USA. 1990;87:5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yim M B, Chock P B, Stadtman E R. J Biol Chem. 1993;268:4099–4105. [PubMed] [Google Scholar]
- 39.Wiedau-Pazos M, Goto J J, Rabizadeh S, Gralla E B, Roe J A, Lee M K, Valentine J S, Bredesen D E. Science. 1996;271:515–518. doi: 10.1126/science.271.5248.515. [DOI] [PubMed] [Google Scholar]
- 40.Yim M B, Kang J-H, Yim H-S, Kwak H-S, Chock P B, Stadtman E R. Proc Natl Acad Sci USA. 1996;93:5709–5714. doi: 10.1073/pnas.93.12.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epstein C J, Avraham K B, Lovett M, Smith S, Elroy-Stein O, Rotman G, Bry C, Groner Y. Proc Natl Acad Sci USA. 1987;84:8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avraham K B. Ph.D. thesis. Rehovot, Israel: The Weizmann Institute; 1990. pp. 32–33. [Google Scholar]
- 43.White C W, Ngugen D H, Suzuki K, Tanaguchi N, Rusakow L S, Avraham K B, Groner Y. Free Radical Biol Med. 1993;15:629–636. doi: 10.1016/0891-5849(93)90166-r. [DOI] [PubMed] [Google Scholar]
- 44.Halliwell B, Gutteridge J M C. Free Radicals in Biology and Medicine. 2nd Ed. Oxford: Clarendon; 1989. [DOI] [PubMed] [Google Scholar]
- 45.Shechter Y, Shisheva A, Lazar R, Libman J, Shanzer A. Biochemistry. 1992;31:2063–2068. doi: 10.1021/bi00122a024. [DOI] [PubMed] [Google Scholar]
- 46.Hall E D, Wolf D L, Althaus J S, VonVoigtlander P F. Brain Res. 1987;435:174–180. doi: 10.1016/0006-8993(87)91599-x. [DOI] [PubMed] [Google Scholar]
- 47.Buettner G R. Free Radical Biol Med. 1987;3:259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- 48.Lathe R. Trends Neurosci. 1996;19:183–186. doi: 10.1016/s0166-2236(96)20022-0. [DOI] [PubMed] [Google Scholar]