Abstract
The expression of the nrd operon encoding ribonucleotide reductase in Escherichia coli has been shown to be cell cycle regulated. To identify the cis-acting elements required for the cell cycle regulation of the nrd promoter, different 5' deletions as well as site-directed mutations were translationally fused to a lacZ reporter gene. The expression of beta-galactosidase from these nrd-lacZ fusions in single-copy plasmids was determined with synchronously growing cultures obtained by repeated phosphate starvation as well as with exponentially growing cultures by flow cytometry analysis. Although Fis and DnaA, two regulatory proteins that bind at multiple sites on the E. coli chromosome, have been found to regulate the nrd promoter, the results in this study demonstrated that neither Fis nor DnaA was required for nrd cell cycle regulation. A cis-acting upstream AT-rich sequence was found to be required for the cell cycle regulation. This sequence could be replaced by a different sequence that maintained the AT richness. A flow cytometry analysis that combined specific immunofluorescent staining of beta-galactosidase with a DNA-specific stain was developed and employed to study the nrd promoter activity in cells at specific cell cycle positions. The results of the flow cytometry analysis confirmed the results obtained from studies with synchronized cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. J. Gene expression. Dialogue with the cell cycle. Nature. 1992 Jan 30;355(6359):393–394. doi: 10.1038/355393a0. [DOI] [PubMed] [Google Scholar]
- Andrews B. J., Herskowitz I. Regulation of cell cycle-dependent gene expression in yeast. J Biol Chem. 1990 Aug 25;265(24):14057–14060. [PubMed] [Google Scholar]
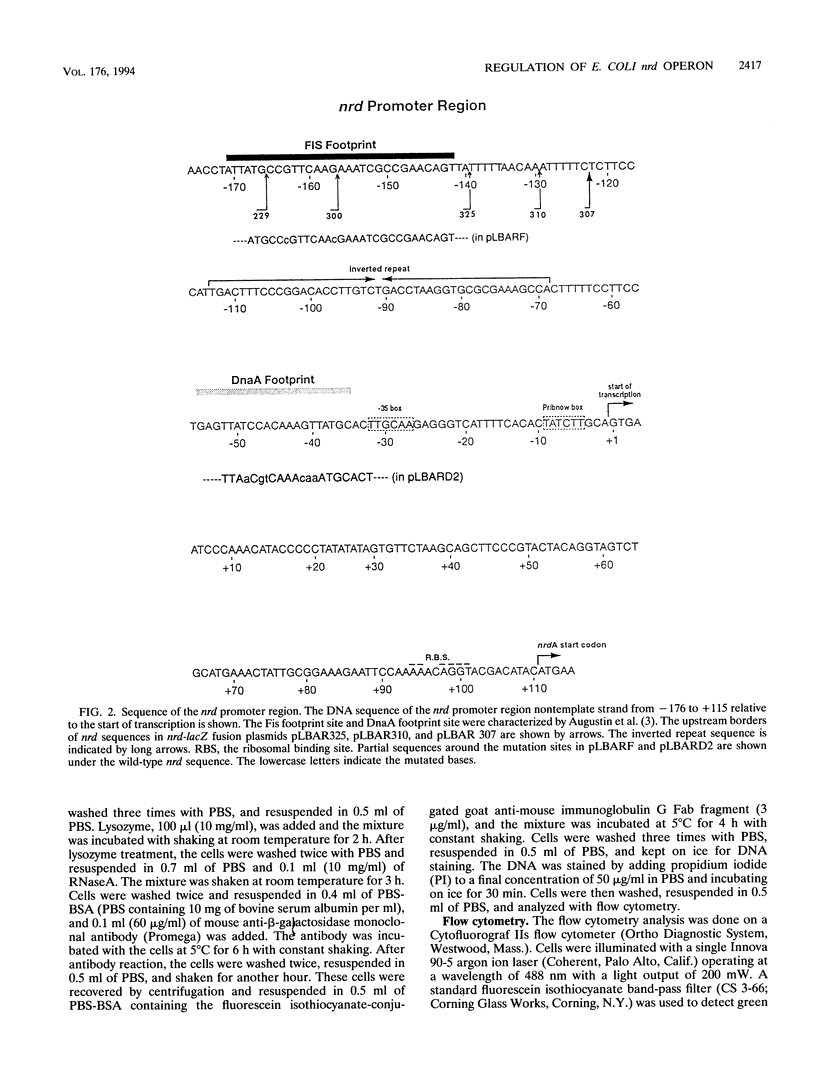
- Augustin L. B., Jacobson B. A., Fuchs J. A. Escherichia coli Fis and DnaA proteins bind specifically to the nrd promoter region and affect expression of an nrd-lac fusion. J Bacteriol. 1994 Jan;176(2):378–387. doi: 10.1128/jb.176.2.378-387.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block D. E., Eitzman P. D., Wangensteen J. D., Srienc F. Slit scanning of Saccharomyces cerevisiae cells: quantification of asymmetric cell division and cell cycle progression in asynchronous culture. Biotechnol Prog. 1990 Nov-Dec;6(6):504–512. doi: 10.1021/bp00006a015. [DOI] [PubMed] [Google Scholar]
- Bosch L., Nilsson L., Vijgenboom E., Verbeek H. FIS-dependent trans-activation of tRNA and rRNA operons of Escherichia coli. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):293–301. doi: 10.1016/0167-4781(90)90184-4. [DOI] [PubMed] [Google Scholar]
- Boye E., Løbner-Olesen A. Bacterial growth control studied by flow cytometry. Res Microbiol. 1991 Feb-Apr;142(2-3):131–135. doi: 10.1016/0923-2508(91)90020-b. [DOI] [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Carlson J., Fuchs J. A., Messing J. Primary structure of the Escherichia coli ribonucleoside diphosphate reductase operon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4294–4297. doi: 10.1073/pnas.81.14.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzman P. D., Hendrick J. L., Srienc F. Quantitative immunofluorescence in single Saccharomyces cerevisiae cells. Cytometry. 1989 Jul;10(4):475–483. doi: 10.1002/cyto.990100417. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Davis R. W. Two genes differentially regulated in the cell cycle and by DNA-damaging agents encode alternative regulatory subunits of ribonucleotide reductase. Genes Dev. 1990 May;4(5):740–751. doi: 10.1101/gad.4.5.740. [DOI] [PubMed] [Google Scholar]
- Engström Y., Eriksson S., Jildevik I., Skog S., Thelander L., Tribukait B. Cell cycle-dependent expression of mammalian ribonucleotide reductase. Differential regulation of the two subunits. J Biol Chem. 1985 Aug 5;260(16):9114–9116. [PubMed] [Google Scholar]
- Engström Y., Rozell B. Immunocytochemical evidence for the cytoplasmic localization and differential expression during the cell cycle of the M1 and M2 subunits of mammalian ribonucleotide reductase. EMBO J. 1988 Jun;7(6):1615–1620. doi: 10.1002/j.1460-2075.1988.tb02987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sarabia M. J., Fantes P. A. Ribonucleotide reductase and its regulation during the cell cycle. Trends Genet. 1990 Sep;6(9):275–276. doi: 10.1016/0168-9525(90)90214-q. [DOI] [PubMed] [Google Scholar]
- Filpula D., Fuchs J. A. Regulation of ribonucleoside diphosphate reductase synthesis in Escherichia coli: increased enzyme synthesis as a result of inhibition of deoxyribonucleic acid synthesis. J Bacteriol. 1977 Apr;130(1):107–113. doi: 10.1128/jb.130.1.107-113.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filpula D., Fuchs J. A. Regulation of the synthesis of ribonucleoside diphosphate reductase in Escherichia coli: specific activity of the enzyme in relationship to perturbations of DNA replication. J Bacteriol. 1978 Aug;135(2):429–435. doi: 10.1128/jb.135.2.429-435.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filutowicz M., Ross W., Wild J., Gourse R. L. Involvement of Fis protein in replication of the Escherichia coli chromosome. J Bacteriol. 1992 Jan;174(2):398–407. doi: 10.1128/jb.174.2.398-407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O. Mapping of nrdA and nrdB in Escherichia coli K-12. J Bacteriol. 1976 Dec;128(3):810–814. doi: 10.1128/jb.128.3.810-814.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido T., Sánchez M., Palacios P., Aldea M., Vicente M. Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J. 1993 Oct;12(10):3957–3965. doi: 10.1002/j.1460-2075.1993.tb06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H., Egan J. B., Roth A., Messer W. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli. Nucleic Acids Res. 1991 Aug 11;19(15):4167–4172. doi: 10.1093/nar/19.15.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke P. D., Fuchs J. A. Regulation of ribonucleoside diphosphate reductase mRNA synthesis in Escherichia coli. J Bacteriol. 1983 Jun;154(3):1040–1045. doi: 10.1128/jb.154.3.1040-1045.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Ogura T., Niki H., Ichinose C., Mori H. Positioning of replicated chromosomes in Escherichia coli. J Bacteriol. 1990 Jan;172(1):31–39. doi: 10.1128/jb.172.1.31-39.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., Gottesman S. Cell-division control in Escherichia coli: specific induction of the SOS function SfiA protein is sufficient to block septation. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4490–4494. doi: 10.1073/pnas.81.14.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino F., Matsuhashi M. Peptidoglycan synthetic enzyme activities of highly purified penicillin-binding protein 3 in Escherichia coli: a septum-forming reaction sequence. Biochem Biophys Res Commun. 1981 Aug 14;101(3):905–911. doi: 10.1016/0006-291x(81)91835-0. [DOI] [PubMed] [Google Scholar]
- Jaffé A., D'Ari R., Norris V. SOS-independent coupling between DNA replication and cell division in Escherichia coli. J Bacteriol. 1986 Jan;165(1):66–71. doi: 10.1128/jb.165.1.66-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Bruist M. F., Simon M. I. Host protein requirements for in vitro site-specific DNA inversion. Cell. 1986 Aug 15;46(4):531–539. doi: 10.1016/0092-8674(86)90878-0. [DOI] [PubMed] [Google Scholar]
- Kepes F., Kepes A. Long - lasting synchrony of the division of enteric bacteria. Biochem Biophys Res Commun. 1981 Apr 15;99(3):761–767. doi: 10.1016/0006-291x(81)91230-4. [DOI] [PubMed] [Google Scholar]
- Kepes F., Kepes A. Synchronisation automatique de la croissance de Escherichia coli. Ann Microbiol (Paris) 1980 Jan-Feb;131(1):3–16. [PubMed] [Google Scholar]
- Kline B. C., Kogoma T., Tam J. E., Shields M. S. Requirement of the Escherichia coli dnaA gene product for plasmid F maintenance. J Bacteriol. 1986 Oct;168(1):440–443. doi: 10.1128/jb.168.1.440-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Kahmann R. Purification and properties of the Escherichia coli host factor required for inversion of the G segment in bacteriophage Mu. J Biol Chem. 1986 Nov 25;261(33):15673–15678. [PubMed] [Google Scholar]
- Larsen J. E., Albrechtsen B., Valentin-Hansen P. Analysis of the terminator region after the deoCABD operon of Escherichia coli K-12 using a new class of single copy number operon-fusion vectors. Nucleic Acids Res. 1987 Jul 10;15(13):5125–5140. doi: 10.1093/nar/15.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowndes N. F., Johnson A. L., Johnston L. H. Coordination of expression of DNA synthesis genes in budding yeast by a cell-cycle regulated trans factor. Nature. 1991 Mar 21;350(6315):247–250. doi: 10.1038/350247a0. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. Regulation of cell division in E. coli. Trends Genet. 1990 Jan;6(1):22–25. doi: 10.1016/0168-9525(90)90045-8. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Nir R., Yisraeli Y., Lamed R., Sahar E. Flow cytometry sorting of viable bacteria and yeasts according to beta-galactosidase activity. Appl Environ Microbiol. 1990 Dec;56(12):3861–3866. doi: 10.1128/aem.56.12.3861-3866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Bernander R., Dasgupta S. The Escherichia coli cell cycle: one cycle or multiple independent processes that are co-ordinated? Mol Microbiol. 1991 Apr;5(4):769–774. doi: 10.1111/j.1365-2958.1991.tb00747.x. [DOI] [PubMed] [Google Scholar]
- Ross W., Thompson J. F., Newlands J. T., Gourse R. L. E.coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990 Nov;9(11):3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. A., Yajko D. M., Nassos P. S., Hyun W. C., Fulwyler M. J., Hadley W. K. Detection and analysis by dual-laser flow cytometry of bacteriophage T4 DNA inside Escherichia coli. Cytometry. 1991;12(2):167–171. doi: 10.1002/cyto.990120211. [DOI] [PubMed] [Google Scholar]
- Skarstad K., von Meyenburg K., Hansen F. G., Boye E. Coordination of chromosome replication initiation in Escherichia coli: effects of different dnaA alleles. J Bacteriol. 1988 Feb;170(2):852–858. doi: 10.1128/jb.170.2.852-858.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srienc F., Dien B. S. Kinetics of the cell cycle of Saccharomyces cerevisiae. Ann N Y Acad Sci. 1992 Oct 13;665:59–71. doi: 10.1111/j.1749-6632.1992.tb42574.x. [DOI] [PubMed] [Google Scholar]
- Steen H. B., Boye E. Bacterial growth studied by flow cytometry. Cytometry. 1980 Jul;1(1):32–36. doi: 10.1002/cyto.990010108. [DOI] [PubMed] [Google Scholar]
- Steen H. B., Boye E. Escherichia coli growth studied by dual-parameter flow cytophotometry. J Bacteriol. 1981 Feb;145(2):1091–1094. doi: 10.1128/jb.145.2.1091-1094.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen H. B., Skarstad K., Boye E. DNA measurements of bacteria. Methods Cell Biol. 1990;33:519–526. doi: 10.1016/s0091-679x(08)60551-8. [DOI] [PubMed] [Google Scholar]
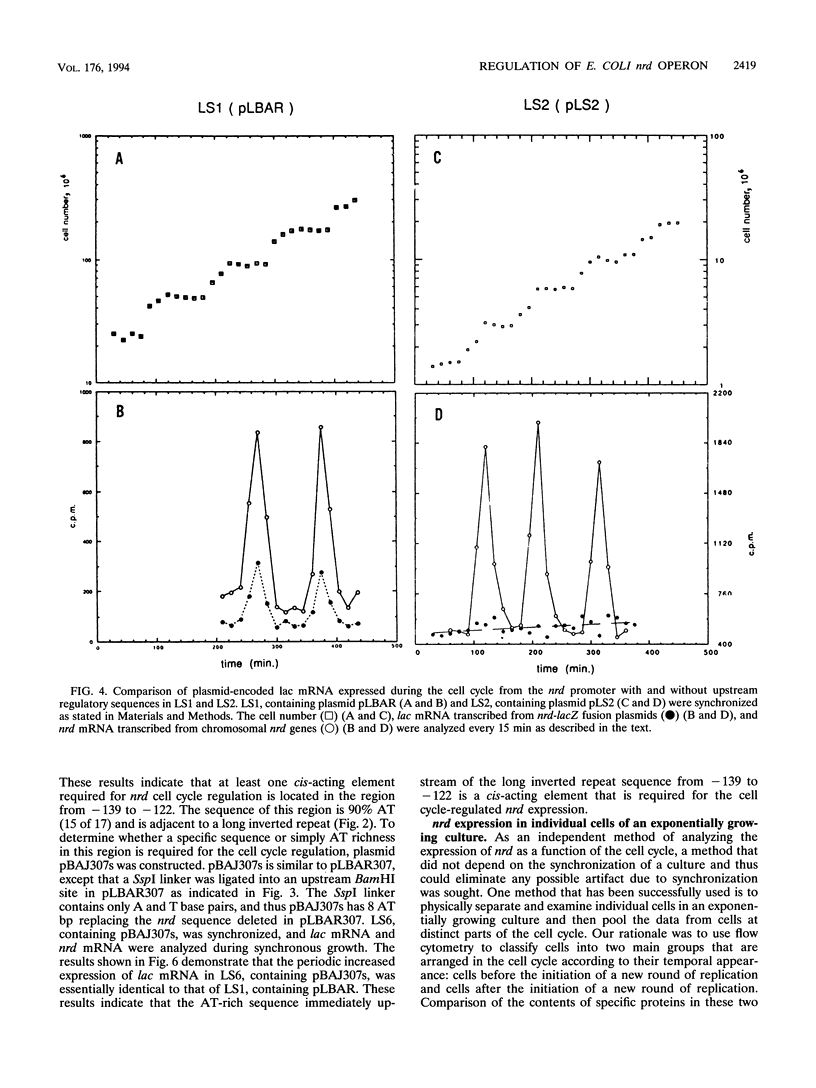
- Sun L., Fuchs J. A. Escherichia coli ribonucleotide reductase expression is cell cycle regulated. Mol Biol Cell. 1992 Oct;3(10):1095–1105. doi: 10.1091/mbc.3.10.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Selzer G. Initiation of DNA synthesis in Escherichia coli. Annu Rev Biochem. 1979;48:999–1034. doi: 10.1146/annurev.bi.48.070179.005031. [DOI] [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Regulation of the operon encoding ribonucleotide reductase in Escherichia coli: evidence for both positive and negative control. EMBO J. 1986 May;5(5):1077–1085. doi: 10.1002/j.1460-2075.1986.tb04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Regulation of the operon encoding ribonucleotide reductase: role of the negative sites in nrd repression. J Bacteriol. 1990 Apr;172(4):1711–1718. doi: 10.1128/jb.172.4.1711-1718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Massy B., Fayet O., Kogoma T. Multiple origin usage for DNA replication in sdrA(rnh) mutants of Escherichia coli K-12. Initiation in the absence of oriC. J Mol Biol. 1984 Sep 15;178(2):227–236. doi: 10.1016/0022-2836(84)90141-4. [DOI] [PubMed] [Google Scholar]
- von Freiesleben U., Rasmussen K. V. DNA replication in Escherichia coli gyrB(Ts) mutants analysed by flow cytometry. Res Microbiol. 1991 Feb-Apr;142(2-3):223–227. doi: 10.1016/0923-2508(91)90034-8. [DOI] [PubMed] [Google Scholar]
- von Freiesleben U., Rasmussen K. V. The level of supercoiling affects the regulation of DNA replication in Escherichia coli. Res Microbiol. 1992 Sep;143(7):655–663. doi: 10.1016/0923-2508(92)90060-2. [DOI] [PubMed] [Google Scholar]



