Abstract
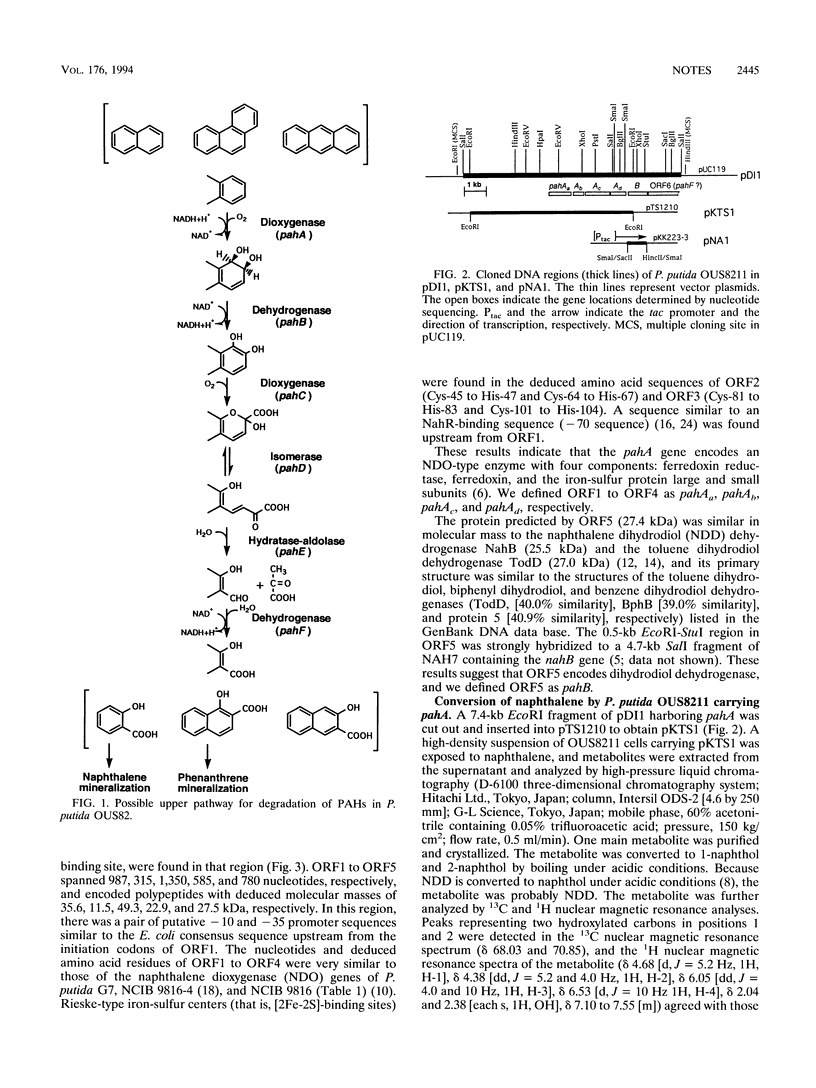
Naphthalene and phenanthrene are transformed by enzymes encoded by the pah gene cluster of Pseudomonas putida OUS82. The pahA and pahB genes, which encode the first and second enzymes, dioxygenase and cis-dihydrodiol dehydrogenase, respectively, were identified and sequenced. The DNA sequences showed that pahA and pahB were clustered and that pahA consisted of four cistrons, pahAa, pahAb, pahAc, and pahAd, which encode ferredoxin reductase, ferredoxin, and two subunits of the iron-sulfur protein, respectively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. I., Evans W. C. Oxidative metabolism of naphthalene by soil pseudomonads. The ring-fission mechanism. Biochem J. 1964 May;91(2):251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denome S. A., Stanley D. C., Olson E. S., Young K. D. Metabolism of dibenzothiophene and naphthalene in Pseudomonas strains: complete DNA sequence of an upper naphthalene catabolic pathway. J Bacteriol. 1993 Nov;175(21):6890–6901. doi: 10.1128/jb.175.21.6890-6901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. W., Gunsalus I. C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973 Jun;114(3):974–979. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. W., Chapman P. J. Bacterial metabolism of naphthalene: construction and use of recombinant bacteria to study ring cleavage of 1,2-dihydroxynaphthalene and subsequent reactions. J Bacteriol. 1992 Dec;174(23):7542–7554. doi: 10.1128/jb.174.23.7542-7554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T., Laborde A. L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982 Mar;149(3):948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Jeffrey A. M., Gibson D. T. Cis-1,2-dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971 Jan;142(1):394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- Kiyohara H., Torigoe S., Kaida N., Asaki T., Iida T., Hayashi H., Takizawa N. Cloning and characterization of a chromosomal gene cluster, pah, that encodes the upper pathway for phenanthrene and naphthalene utilization by Pseudomonas putida OUS82. J Bacteriol. 1994 Apr;176(8):2439–2443. doi: 10.1128/jb.176.8.2439-2443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkela S., Lehväslaiho H., Palva E. T., Teeri T. H. Cloning, nucleotide sequence and characterization of genes encoding naphthalene dioxygenase of Pseudomonas putida strain NCIB9816. Gene. 1988 Dec 20;73(2):355–362. doi: 10.1016/0378-1119(88)90500-8. [DOI] [PubMed] [Google Scholar]
- Patel T. R., Gibson D. T. Purification and propeties of (plus)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J Bacteriol. 1974 Sep;119(3):879–888. doi: 10.1128/jb.119.3.879-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins E. J., Gordon M. P., Caceres O., Lurquin P. F. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990 May;172(5):2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. E., Gibson D. T. Purification and properties of cis-toluene dihydrodiol dehydrogenase from Pseudomonas putida. J Bacteriol. 1977 Jun;130(3):1117–1124. doi: 10.1128/jb.130.3.1117-1124.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. A., Poser E. F. Demonstration, characterization, and mutational analysis of NahR protein binding to nah and sal promoters. J Bacteriol. 1989 Feb;171(2):837–846. doi: 10.1128/jb.171.2.837-846.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Y., Seed B. Electrolyte gradient gels for DNA sequencing. Biotechniques. 1988 Nov-Dec;6(10):942–944. [PubMed] [Google Scholar]
- Simon M. J., Osslund T. D., Saunders R., Ensley B. D., Suggs S., Harcourt A., Suen W. C., Cruden D. L., Gibson D. T., Zylstra G. J. Sequences of genes encoding naphthalene dioxygenase in Pseudomonas putida strains G7 and NCIB 9816-4. Gene. 1993 May 15;127(1):31–37. doi: 10.1016/0378-1119(93)90613-8. [DOI] [PubMed] [Google Scholar]
- Taira K., Hayase N., Arimura N., Yamashita S., Miyazaki T., Furukawa K. Cloning and nucleotide sequence of the 2,3-dihydroxybiphenyl dioxygenase gene from the PCB-degrading strain of Pseudomonas paucimobilis Q1. Biochemistry. 1988 May 31;27(11):3990–3996. doi: 10.1021/bi00411a015. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yen K. M., Gunsalus I. C. Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci U S A. 1982 Feb;79(3):874–878. doi: 10.1073/pnas.79.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K. M., Serdar C. M. Genetics of naphthalene catabolism in pseudomonads. Crit Rev Microbiol. 1988;15(3):247–268. doi: 10.3109/10408418809104459. [DOI] [PubMed] [Google Scholar]
- You I. S., Ghosal D., Gunsalus I. C. Nucleotide sequence of plasmid NAH7 gene nahR and DNA binding of the nahR product. J Bacteriol. 1988 Dec;170(12):5409–5415. doi: 10.1128/jb.170.12.5409-5415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


