Abstract
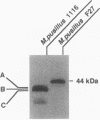
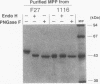
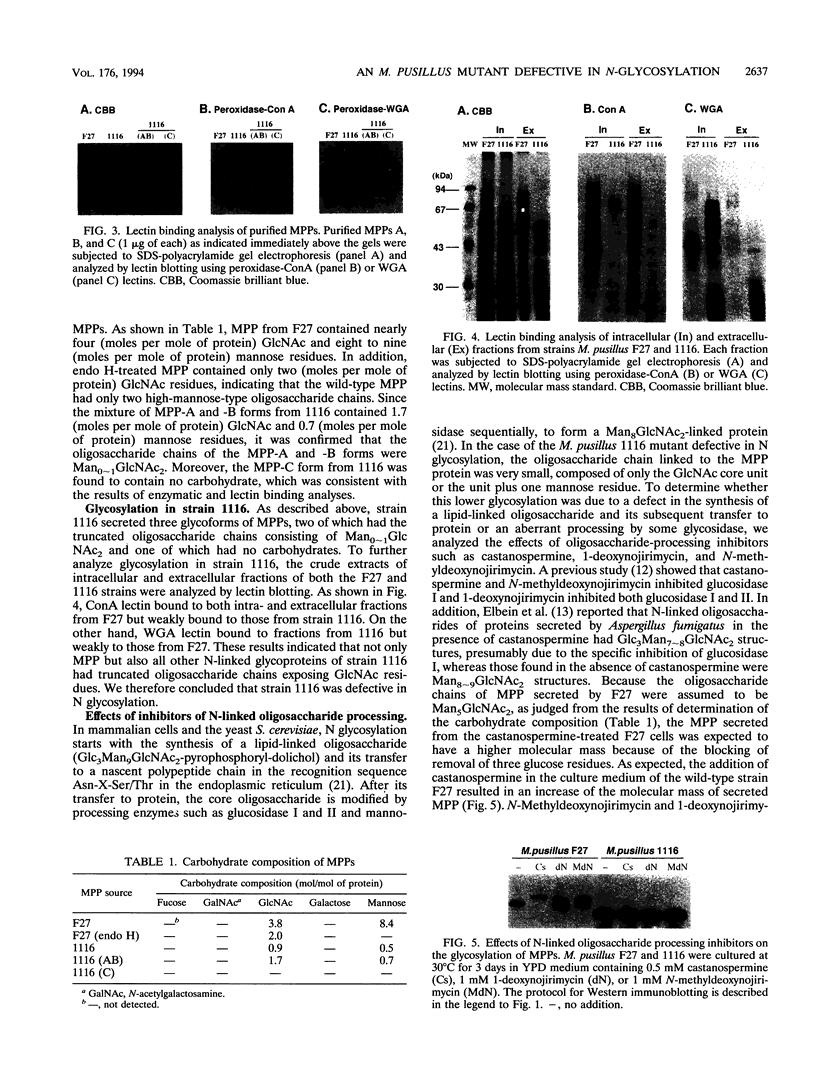
A Mucor pusillus mutant defective in asparagine-linked glycosylation was found in our stock cultures. This mutant, designated 1116, secreted aspartic proteinase (MPP) in a less-glycosylated form than that secreted by the wild-type strain. Analysis of enzyme susceptibility, lectin binding, and carbohydrate composition indicated that this mutant secreted three glycoforms of MPPs, one of which contained no carbohydrate; the other two had truncated asparagine-linked oligosaccharide chains such as Man0-1GlcNAc2. Further analysis using oligosaccharide processing inhibitors, such as castanospermine, 1-deoxynojirimycin and N-methyldeoxynojirimycin, suggested that MPPs in the mutant were glycosylated through a transfer of the truncated lipid-linked oligosaccharides, Man0-1GlcNAc2, to the MPP protein but not through an aberrant processing. In addition, genetic studies with forced primary heterokaryons indicated that the mutation in strain 1116 was recessive.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeijon C., Hirschberg C. B. Topography of glycosylation reactions in the endoplasmic reticulum. Trends Biochem Sci. 1992 Jan;17(1):32–36. doi: 10.1016/0968-0004(92)90424-8. [DOI] [PubMed] [Google Scholar]
- Abeijon C., Hirschberg C. B. Topography of initiation of N-glycosylation reactions. J Biol Chem. 1990 Aug 25;265(24):14691–14695. [PubMed] [Google Scholar]
- Aikawa J., Yamashita T., Nishiyama M., Horinouchi S., Beppu T. Effects of glycosylation on the secretion and enzyme activity of Mucor rennin, an aspartic proteinase of Mucor pusillus, produced by recombinant yeast. J Biol Chem. 1990 Aug 15;265(23):13955–13959. [PubMed] [Google Scholar]
- Arima K., Yu J., Iwasaki S., Tamura G. Milk-clotting Enzyme from Microorganisms: V. Purification and Crystallization of Mucor Rennin from Mucor pusillus var. Lindt. Appl Microbiol. 1968 Nov;16(11):1727–1733. doi: 10.1128/am.16.11.1727-1733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudys M., Foundling S., Pavlík M., Blundell T., Kostka V. Protein chemical characterization of Mucor pusillus aspartic proteinase. Amino acid sequence homology with the other aspartic proteinases, disulfide bond arrangement and site of carbohydrate attachment. FEBS Lett. 1988 Aug 1;235(1-2):271–274. doi: 10.1016/0014-5793(88)81277-8. [DOI] [PubMed] [Google Scholar]
- Brändli A. W. Mammalian glycosylation mutants as tools for the analysis and reconstitution of protein transport. Biochem J. 1991 May 15;276(Pt 1):1–12. doi: 10.1042/bj2760001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y., Yamagata Y., Nakajima T., Ichishima E. A new high-mannose type N-linked oligosaccharide from Aspergillus carboxypeptidase. Biosci Biotechnol Biochem. 1992 Aug;56(8):1371–1372. doi: 10.1271/bbb.56.1371. [DOI] [PubMed] [Google Scholar]
- Chu F. K. Requirements of cleavage of high mannose oligosaccharides in glycoproteins by peptide N-glycosidase F. J Biol Chem. 1986 Jan 5;261(1):172–177. [PubMed] [Google Scholar]
- Cove D. J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966 Jan 11;113(1):51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Devlin C., Ballou C. E. Identification and characterization of a gene and protein required for glycosylation in the yeast Golgi. Mol Microbiol. 1990 Nov;4(11):1993–2001. doi: 10.1111/j.1365-2958.1990.tb02049.x. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Mitchell M., Molyneux R. J. Effect of castanospermine on the structure and secretion of glycoprotein enzymes in Aspergillus fumigatus. J Bacteriol. 1984 Oct;160(1):67–75. doi: 10.1128/jb.160.1.67-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon B., Esmon P. C., Schekman R. Early steps in processing of yeast glycoproteins. J Biol Chem. 1984 Aug 25;259(16):10322–10327. [PubMed] [Google Scholar]
- Hardy M. R., Townsend R. R., Lee Y. C. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem. 1988 Apr;170(1):54–62. doi: 10.1016/0003-2697(88)90089-9. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Snider M. D. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Temperature-sensitive yeast mutants deficient in asparagine-linked glycosylation. J Biol Chem. 1982 Mar 25;257(6):3203–3210. [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Yeast mutants deficient in protein glycosylation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7466–7470. doi: 10.1073/pnas.80.24.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean E. L. Topographical orientation in microsomal vesicles of the N-acetylglucosaminyltransferases which catalyze the biosynthesis of N-acetylglucosaminylpyrophosphoryldolichol and N-acetylglucosaminyl-N-acetylglucosaminylpyrophosphoryldolichol. J Biol Chem. 1991 Jan 15;266(2):942–946. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Bergh M. L., Jackson B. J. Protein glycosylation in yeast. Annu Rev Biochem. 1987;56:915–944. doi: 10.1146/annurev.bi.56.070187.004411. [DOI] [PubMed] [Google Scholar]
- Murakami K., Aikawa J., Horinouchi S., Beppu T. Characterization of an aspartic proteinase of Mucor pusillus expressed in Aspergillus oryzae. Mol Gen Genet. 1993 Nov;241(3-4):312–318. doi: 10.1007/BF00284683. [DOI] [PubMed] [Google Scholar]
- Sharma C. B., Lehle L., Tanner W. N-Glycosylation of yeast proteins. Characterization of the solubilized oligosaccharyl transferase. Eur J Biochem. 1981 May;116(1):101–108. doi: 10.1111/j.1432-1033.1981.tb05306.x. [DOI] [PubMed] [Google Scholar]
- Takegawa K., Kawasaki N., Iwahara S., Yamamoto K., Tochikura T., Mikami B., Morita Y. Primary structure of an N-linked sugar chain derived from glucoamylase of Rhizopus niveus. Biochim Biophys Acta. 1989 Jan 27;990(1):98–100. doi: 10.1016/s0304-4165(89)80018-2. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Tonouchi N., Shoun H., Uozumi T., Beppu T. Cloning and sequencing of a gene for Mucor rennin, an aspartate protease from Mucor pusillus. Nucleic Acids Res. 1986 Oct 10;14(19):7557–7568. doi: 10.1093/nar/14.19.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Tonouchi N., Uozumi T., Beppu T. Secretion of Mucor rennin, a fungal aspartic protease of Mucor pusillus, by recombinant yeast cells. Mol Gen Genet. 1987 Dec;210(3):462–467. doi: 10.1007/BF00327198. [DOI] [PubMed] [Google Scholar]