Abstract
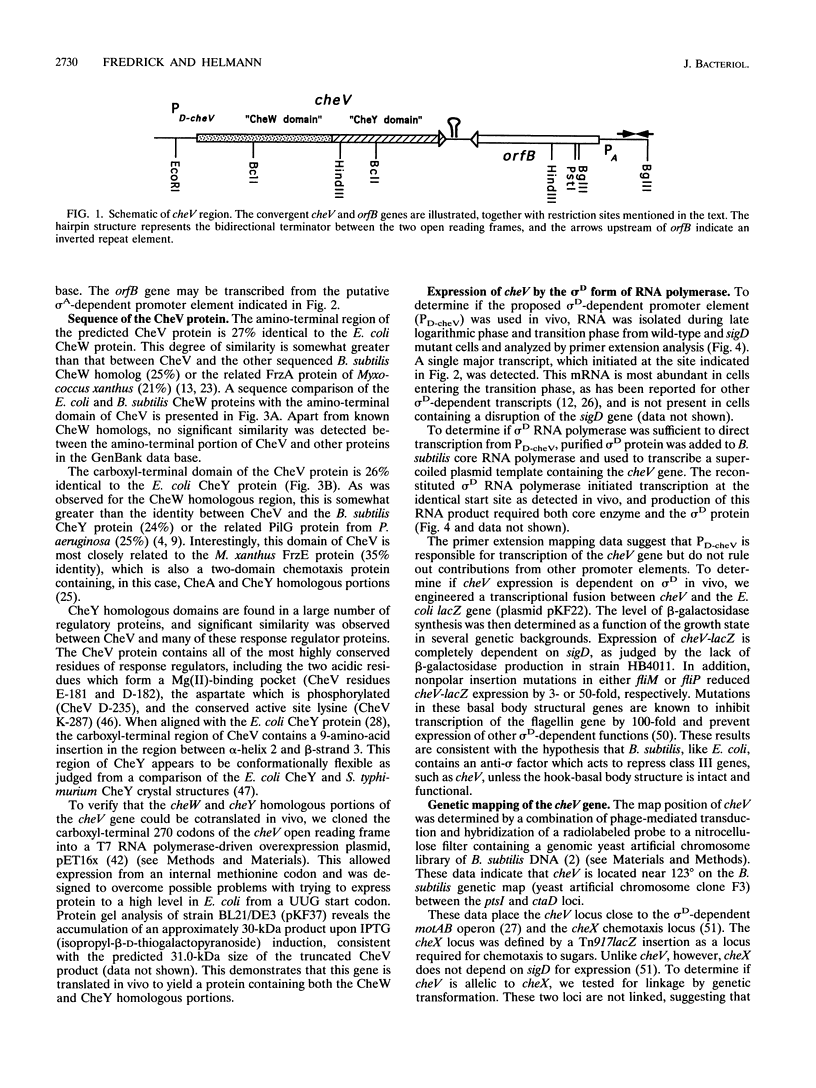
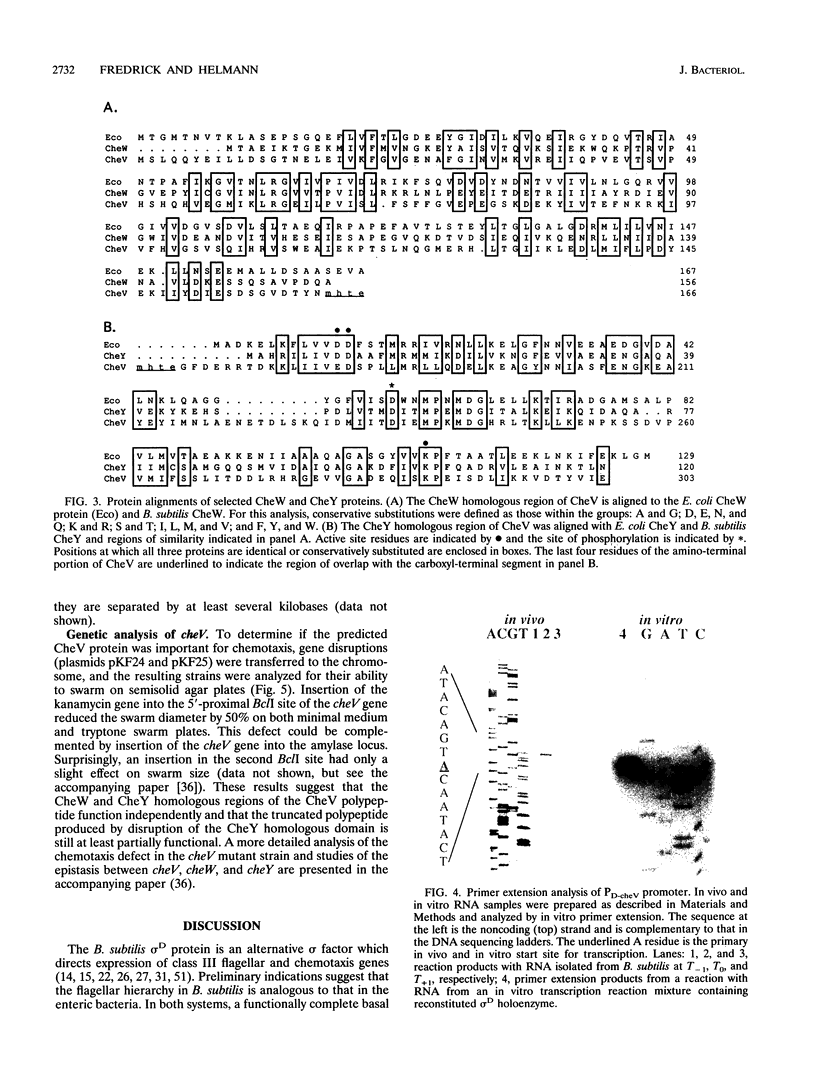
The alternative sigma factor, sigma D, activates the expression of genes required for chemotaxis and motility in Bacillus subtilis, including those encoding flagellin, hook-associated proteins, and the motor proteins. The sigma D protein is encoded in a large operon which also encodes the structural proteins for the basal body and homologs of the enteric CheW, CheY, CheA, and CheB chemotaxis proteins. We report the identification and molecular characterization of a novel chemotaxis gene, cheV. The predicted CheV gene product contains an amino-terminal CheW homologous domain linked to a response regulator domain of the CheY family, suggesting that either or both of these functions are duplicated. Transcription of cheV initiates from a sigma D-dependent promoter element both in vivo and in vitro, and expression of a cheV-lacZ fusion is completely dependent on sigD. Expression is repressed by nonpolar mutations in structural genes for the basal body, fliM or fliP, indicating that cheV belongs to class III in the B. subtilis flagellar hierarchy. The cheV locus is monocistronic and is located at 123 degrees on the B. subtilis genetic map near the previously defined cheX locus. A cheV mutant strain is motile but impaired in chemotaxis on swarm plates. Surprisingly, an insertion in the CheW homologous domain leads to a more severe defect than an insertion in the CheY homologous domain. The presence of dual pathways for chemotactic signal transduction is consistent with the residual signaling observed in previous studies of cheW mutants (D. W. Hanlon, L. Márques-Magaña, P. B. Carpenter, M. J. Chamberlin, and G. W. Ordal, J. Biol. Chem. 267:12055-12060, 1992).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Yoneda Y., Yamane K., Maruo B. Pleiotropic phenomena in autolytic enzyme(s) content, flagellation, and simultaneous hyperproduction of extracellular alpha-amylase and protease in a Bacillus subtilis mutant. J Bacteriol. 1975 Oct;124(1):459–469. doi: 10.1128/jb.124.1.459-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo V., Alvarez E., Zumstein E., Damiani G., Sgaramella V., Ehrlich S. D., Serror P. An ordered collection of Bacillus subtilis DNA segments cloned in yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6047–6051. doi: 10.1073/pnas.90.13.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff D. S., Bourret R. B., Kirsch M. L., Ordal G. W. Purification and characterization of Bacillus subtilis CheY. Biochemistry. 1993 Sep 7;32(35):9256–9261. doi: 10.1021/bi00086a035. [DOI] [PubMed] [Google Scholar]
- Bischoff D. S., Ordal G. W. Bacillus subtilis chemotaxis: a deviation from the Escherichia coli paradigm. Mol Microbiol. 1992 Jan;6(1):23–28. doi: 10.1111/j.1365-2958.1992.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Bischoff D. S., Ordal G. W. Sequence and characterization of Bacillus subtilis CheB, a homolog of Escherichia coli CheY, and its role in a different mechanism of chemotaxis. J Biol Chem. 1991 Jul 5;266(19):12301–12305. [PubMed] [Google Scholar]
- Bohannon D. E., Sonenshein A. L. Positive regulation of glutamate biosynthesis in Bacillus subtilis. J Bacteriol. 1989 Sep;171(9):4718–4727. doi: 10.1128/jb.171.9.4718-4727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J., Plaskitt K. A., Buttner M. J., Méndez C., Helmann J. D. The developmental fate of S. coelicolor hyphae depends upon a gene product homologous with the motility sigma factor of B. subtilis. Cell. 1989 Oct 6;59(1):133–143. doi: 10.1016/0092-8674(89)90876-3. [DOI] [PubMed] [Google Scholar]
- Chen L., James L. P., Helmann J. D. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J Bacteriol. 1993 Sep;175(17):5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzins A. The pilG gene product, required for Pseudomonas aeruginosa pilus production and twitching motility, is homologous to the enteric, single-domain response regulator CheY. J Bacteriol. 1993 Sep;175(18):5934–5944. doi: 10.1128/jb.175.18.5934-5944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegner J. A., Graham D. R., Roth A. F., Dahlquist F. W. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992 Sep 18;70(6):975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- Gillen K. L., Hughes K. T. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J Bacteriol. 1991 Apr;173(7):2301–2310. doi: 10.1128/jb.173.7.2301-2310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Chamberlin M. J. Developmental and genetic regulation of Bacillus subtilis genes transcribed by sigma 28-RNA polymerase. Cell. 1983 Nov;35(1):285–293. doi: 10.1016/0092-8674(83)90231-3. [DOI] [PubMed] [Google Scholar]
- Hanlon D. W., Márquez-Magaña L. M., Carpenter P. B., Chamberlin M. J., Ordal G. W. Sequence and characterization of Bacillus subtilis CheW. J Biol Chem. 1992 Jun 15;267(17):12055–12060. [PubMed] [Google Scholar]
- Helmann J. D. Alternative sigma factors and the regulation of flagellar gene expression. Mol Microbiol. 1991 Dec;5(12):2875–2882. doi: 10.1111/j.1365-2958.1991.tb01847.x. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Masiarz F. R., Chamberlin M. J. Isolation and characterization of the Bacillus subtilis sigma 28 factor. J Bacteriol. 1988 Apr;170(4):1560–1567. doi: 10.1128/jb.170.4.1560-1567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann J. D., Márquez L. M., Chamberlin M. J. Cloning, sequencing, and disruption of the Bacillus subtilis sigma 28 gene. J Bacteriol. 1988 Apr;170(4):1568–1574. doi: 10.1128/jb.170.4.1568-1574.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney T. J., Moran C. P., Jr Genetic evidence for interaction of sigma A with two promoters in Bacillus subtilis. J Bacteriol. 1991 Jun;173(11):3282–3290. doi: 10.1128/jb.173.11.3282-3290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda A., Sekiguchi J. High-level transcription of the major Bacillus subtilis autolysin operon depends on expression of the sigma D gene and is affected by a sin (flaD) mutation. J Bacteriol. 1993 Feb;175(3):795–801. doi: 10.1128/jb.175.3.795-801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonetto M., Gribskov M., Gross C. A. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992 Jun;174(12):3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Parkinson J. S. Genetic analysis of the bacterial flagellum. Trends Genet. 1991 Jun;7(6):196–200. doi: 10.1016/0168-9525(91)90436-t. [DOI] [PubMed] [Google Scholar]
- McBride M. J., Weinberg R. A., Zusman D. R. "Frizzy" aggregation genes of the gliding bacterium Myxococcus xanthus show sequence similarities to the chemotaxis genes of enteric bacteria. Proc Natl Acad Sci U S A. 1989 Jan;86(2):424–428. doi: 10.1073/pnas.86.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter L. L., Wright M. E. Identification of genes encoding components of the swarmer cell flagellar motor and propeller and a sigma factor controlling differentiation of Vibrio parahaemolyticus. J Bacteriol. 1993 Jun;175(11):3361–3371. doi: 10.1128/jb.175.11.3361-3371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary W. R., Zusman D. R. FrzE of Myxococcus xanthus is homologous to both CheA and CheY of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5898–5902. doi: 10.1073/pnas.87.15.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirel D. B., Chamberlin M. J. The Bacillus subtilis flagellin gene (hag) is transcribed by the sigma 28 form of RNA polymerase. J Bacteriol. 1989 Jun;171(6):3095–3101. doi: 10.1128/jb.171.6.3095-3101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirel D. B., Lustre V. M., Chamberlin M. J. An operon of Bacillus subtilis motility genes transcribed by the sigma D form of RNA polymerase. J Bacteriol. 1992 Jul;174(13):4197–4204. doi: 10.1128/jb.174.13.4197-4204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh N., Simon M. I. Nucleotide sequence corresponding to five chemotaxis genes in Escherichia coli. J Bacteriol. 1986 Jan;165(1):161–166. doi: 10.1128/jb.165.1.161-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez L. M., Helmann J. D., Ferrari E., Parker H. M., Ordal G. W., Chamberlin M. J. Studies of sigma D-dependent functions in Bacillus subtilis. J Bacteriol. 1990 Jun;172(6):3435–3443. doi: 10.1128/jb.172.6.3435-3443.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi K., Kutsukake K., Suzuki H., Iino T. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol Gen Genet. 1990 Apr;221(2):139–147. doi: 10.1007/BF00261713. [DOI] [PubMed] [Google Scholar]
- Ohnishi K., Kutsukake K., Suzuki H., Lino T. A novel transcriptional regulation mechanism in the flagellar regulon of Salmonella typhimurium: an antisigma factor inhibits the activity of the flagellum-specific sigma factor, sigma F. Mol Microbiol. 1992 Nov;6(21):3149–3157. doi: 10.1111/j.1365-2958.1992.tb01771.x. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Nettleton D. O., Hoch J. A. Genetics of Bacillus subtilis chemotaxis: isolation and mapping of mutations and cloning of chemotaxis genes. J Bacteriol. 1983 Jun;154(3):1088–1097. doi: 10.1128/jb.154.3.1088-1097.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Karamata D. Genetic analysis of autolysin-deficient and flagellaless mutants of Bacillus subtilis. J Bacteriol. 1984 Dec;160(3):1123–1129. doi: 10.1128/jb.160.3.1123-1129.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R., Bermúdez-Cruz R. M., Chamberlin M. J. Parameters affecting transcription termination by Escherichia coli RNA polymerase. I. Analysis of 13 rho-independent terminators. J Mol Biol. 1992 Mar 5;224(1):31–51. doi: 10.1016/0022-2836(92)90574-4. [DOI] [PubMed] [Google Scholar]
- Rosario M. M., Fredrick K. L., Ordal G. W., Helmann J. D. Chemotaxis in Bacillus subtilis requires either of two functionally redundant CheW homologs. J Bacteriol. 1994 May;176(9):2736–2739. doi: 10.1128/jb.176.9.2736-2739.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. C., Chamberlin M. J. Amplification and isolation of Escherichia coli nusA protein and studies of its effects on in vitro RNA chain elongation. Biochemistry. 1984 Jan 17;23(2):197–203. doi: 10.1021/bi00297a004. [DOI] [PubMed] [Google Scholar]
- Starnbach M. N., Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992 Feb;6(4):459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Surette M. G., McCleary W. R., Stock A. M. Signal transduction in bacterial chemotaxis. J Biol Chem. 1992 Oct 5;267(28):19753–19756. [PubMed] [Google Scholar]
- Strauch M. A., Hoch J. A. Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol Microbiol. 1993 Feb;7(3):337–342. doi: 10.1111/j.1365-2958.1993.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Suh J. W., Boylan S. A., Price C. W. Gene for the alpha subunit of Bacillus subtilis RNA polymerase maps in the ribosomal protein gene cluster. J Bacteriol. 1986 Oct;168(1):65–71. doi: 10.1128/jb.168.1.65-71.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeyar M. A., Zahler S. A. Chromosomal insertions of Tn917 in Bacillus subtilis. J Bacteriol. 1986 Aug;167(2):530–534. doi: 10.1128/jb.167.2.530-534.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellanoweth R. L., Rabinowitz J. C. The influence of ribosome-binding-site elements on translational efficiency in Bacillus subtilis and Escherichia coli in vivo. Mol Microbiol. 1992 May;6(9):1105–1114. doi: 10.1111/j.1365-2958.1992.tb01548.x. [DOI] [PubMed] [Google Scholar]
- Volz K., Matsumura P. Crystal structure of Escherichia coli CheY refined at 1.7-A resolution. J Biol Chem. 1991 Aug 15;266(23):15511–15519. doi: 10.2210/pdb3chy/pdb. [DOI] [PubMed] [Google Scholar]
- Volz K. Structural conservation in the CheY superfamily. Biochemistry. 1993 Nov 9;32(44):11741–11753. doi: 10.1021/bi00095a001. [DOI] [PubMed] [Google Scholar]
- Welch M., Oosawa K., Aizawa S., Eisenbach M. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8787–8791. doi: 10.1073/pnas.90.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi A. R., Bischoff D. S., Ordal G. W. Nucleotide sequence and characterization of a Bacillus subtilis gene encoding a flagellar switch protein. J Bacteriol. 1991 Jan;173(2):710–719. doi: 10.1128/jb.173.2.710-719.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberi A. R., Ying C. W., Parker H. M., Ordal G. W. Transposon Tn917lacZ mutagenesis of Bacillus subtilis: identification of two new loci required for motility and chemotaxis. J Bacteriol. 1990 Dec;172(12):6841–6848. doi: 10.1128/jb.172.12.6841-6848.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]




