Abstract
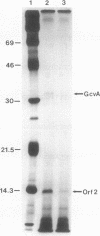
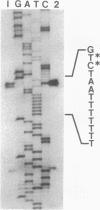
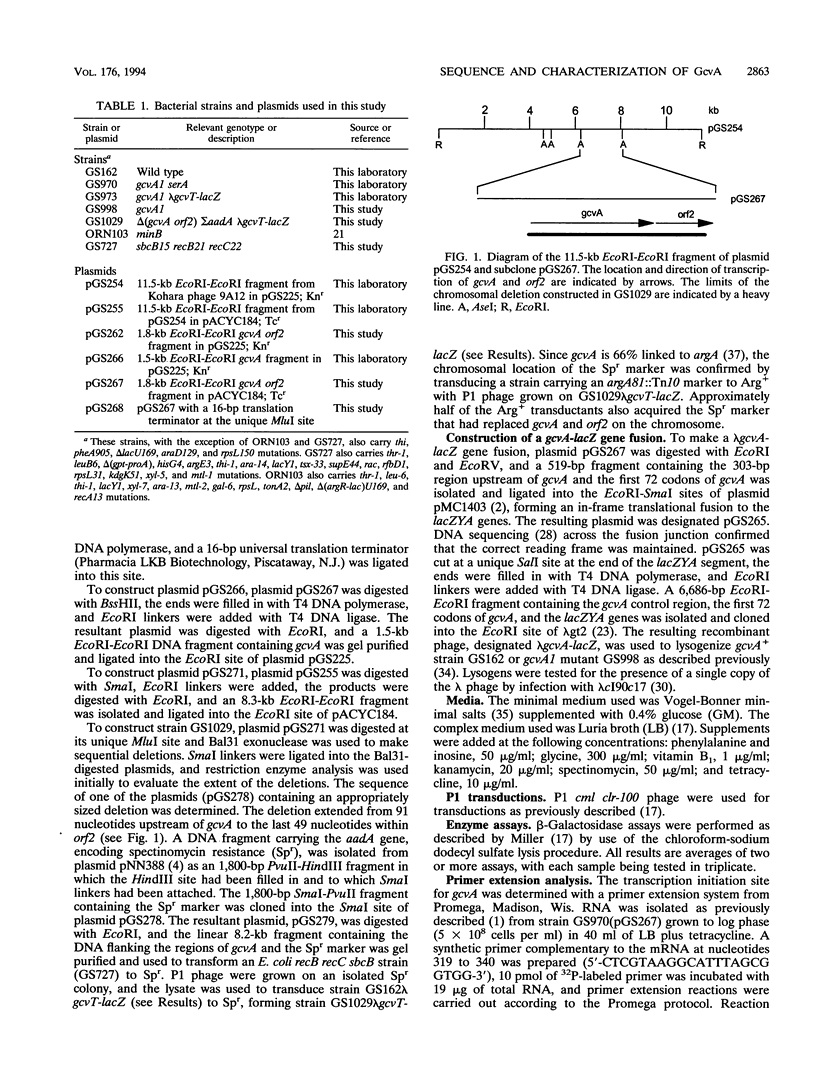
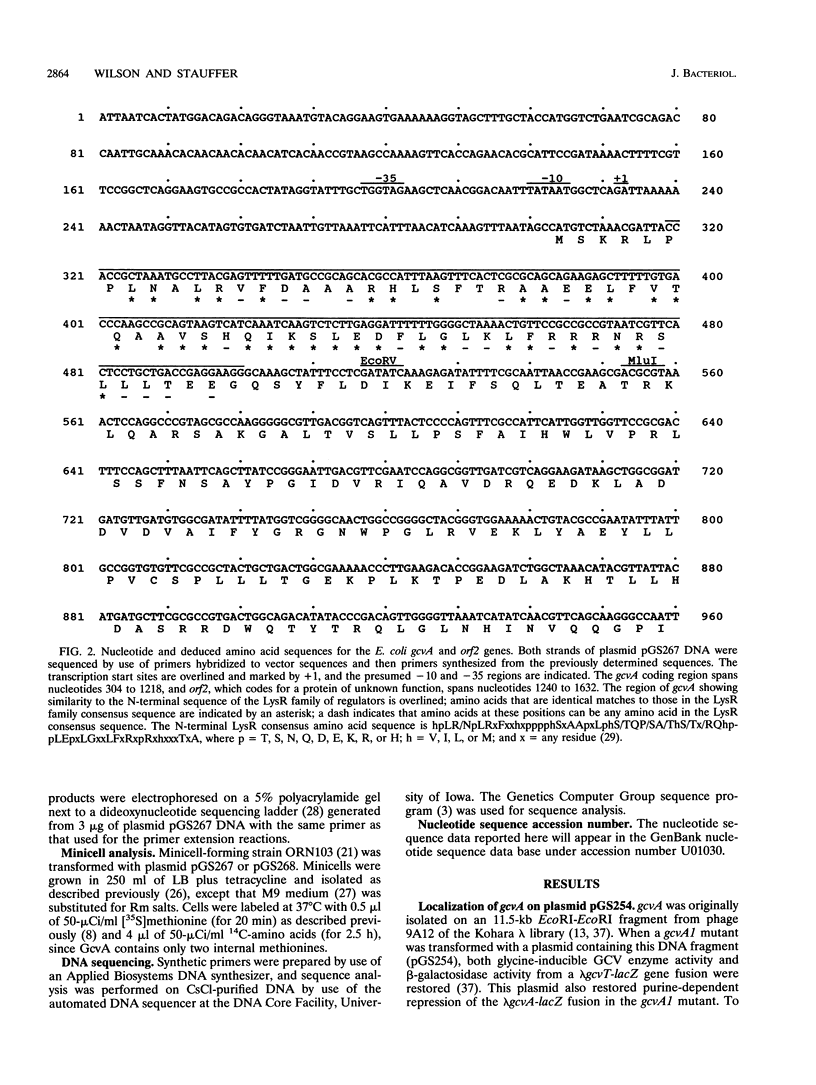
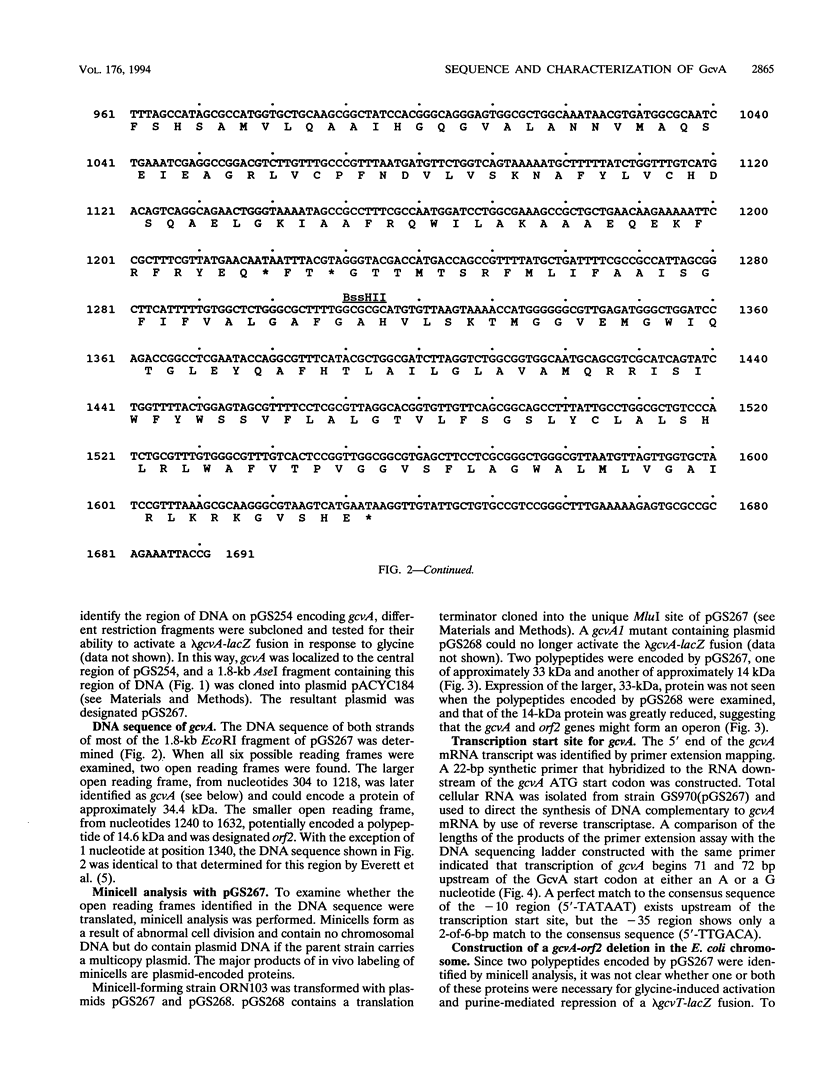
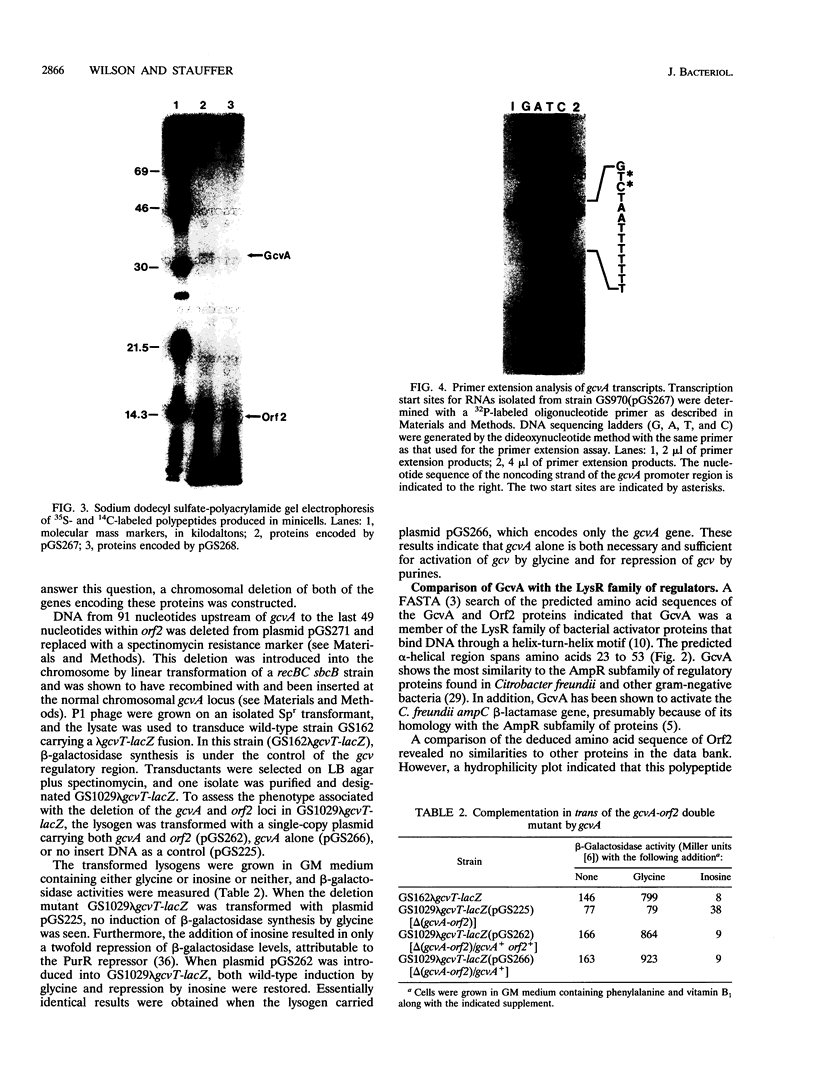
The gene encoding GcvA, the trans-acting regulatory protein for the Escherichia coli glycine cleavage enzyme system, has been sequenced. The gcvA locus contains an open reading frame of 930 nucleotides that could encode a protein with a molecular mass of 34.4 kDa, consistent with the results of minicell analysis indicating that GcvA is a polypeptide of approximately 33 kDa. The deduced amino acid sequence of GcvA revealed that this protein shares similarity with the LysR family of activator proteins. The transcription start site was found to be 72 bp upstream of the presumed translation start site. A chromosomal deletion of gcvA resulted in the inability of cells to activate the expression of a gcvT-lacZ gene fusion when grown in the presence of glycine and an inability to repress gcvT-lacZ expression when grown in the presence of inosine. The regulation of gcvA was examined by constructing a gcvA-lacZ gene fusion in which beta-galactosidase synthesis is under the control of the gcvA regulatory region. Although gcvA expression appears to be autogenously regulated over a two- to threefold range, it is neither induced by glycine nor repressed by inosine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. F., Yanofsky C. The periodicity of RNA polymerase initiations: a new regulatory feature of transcription. Proc Natl Acad Sci U S A. 1968 May;60(1):313–320. doi: 10.1073/pnas.60.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Davis R. W. Position and density effects on repression by stationary and mobile DNA-binding proteins. Genes Dev. 1989 Feb;3(2):185–197. doi: 10.1101/gad.3.2.185. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Okamura K., Motokawa Y. Hydrogen carrier protein from chicken liver: purification, characterization, and role of its prosthetic group, lipolic acid, in the glycine cleavage reaction. Arch Biochem Biophys. 1979 Oct 15;197(2):454–462. doi: 10.1016/0003-9861(79)90267-4. [DOI] [PubMed] [Google Scholar]
- Garnant M. K., Stauffer G. V. Construction and analysis of plasmids containing the Escherichia coli serB gene. Mol Gen Genet. 1984;193(1):72–75. doi: 10.1007/BF00327416. [DOI] [PubMed] [Google Scholar]
- He B., Choi K. Y., Zalkin H. Regulation of Escherichia coli glnB, prsA, and speA by the purine repressor. J Bacteriol. 1993 Jun;175(11):3598–3606. doi: 10.1128/jb.175.11.3598-3606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga K., Kikuchi G. The mitochondrial glycine cleavage system. Functional association of glycine decarboxylase and aminomethyl carrier protein. J Biol Chem. 1980 Dec 25;255(24):11671–11676. [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lin R., D'Ari R., Newman E. B. Lambda placMu insertions in genes of the leucine regulon: extension of the regulon to genes not regulated by leucine. J Bacteriol. 1992 Mar;174(6):1948–1955. doi: 10.1128/jb.174.6.1948-1955.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. A. Structure of the DNA distal to the gene for ribosomal protein S20 in Escherichia coli K12: presence of a strong terminator and an IS1 element. Nucleic Acids Res. 1986 Sep 11;14(17):6965–6981. doi: 10.1093/nar/14.17.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meedel T. H., Pizer L. I. Regulation of one-carbon biosynthesis and utilization in Escherichia coli. J Bacteriol. 1974 Jun;118(3):905–910. doi: 10.1128/jb.118.3.905-910.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G. Glycine metabolism by rat liver mitochondria. Reconstruction of the reversible glycine cleavage system with partially purified protein components. Arch Biochem Biophys. 1974 Oct;164(2):624–633. doi: 10.1016/0003-9861(74)90074-5. [DOI] [PubMed] [Google Scholar]
- Newman E. B., D'Ari R., Lin R. T. The leucine-Lrp regulon in E. coli: a global response in search of a raison d'être. Cell. 1992 Feb 21;68(4):617–619. doi: 10.1016/0092-8674(92)90135-y. [DOI] [PubMed] [Google Scholar]
- Orndorff P. E., Spears P. A., Schauer D., Falkow S. Two modes of control of pilA, the gene encoding type 1 pilin in Escherichia coli. J Bacteriol. 1985 Oct;164(1):321–330. doi: 10.1128/jb.164.1.321-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J., Jagura-Burdzy G., Kredich N. M. DNA sequences of the cysB regions of Salmonella typhimurium and Escherichia coli. J Biol Chem. 1987 May 5;262(13):5999–6005. [PubMed] [Google Scholar]
- Panasenko S. M., Cameron J. R., Davis R. W., Lehman I. R. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977 Apr 8;196(4286):188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- Plamann M. D., Rapp W. D., Stauffer G. V. Escherichia coli K12 mutants defective in the glycine cleavage enzyme system. Mol Gen Genet. 1983;192(1-2):15–20. doi: 10.1007/BF00327641. [DOI] [PubMed] [Google Scholar]
- Rahav-Manor O., Carmel O., Karpel R., Taglicht D., Glaser G., Schuldiner S., Padan E. NhaR, a protein homologous to a family of bacterial regulatory proteins (LysR), regulates nhaA, the sodium proton antiporter gene in Escherichia coli. J Biol Chem. 1992 May 25;267(15):10433–10438. [PubMed] [Google Scholar]
- Reeve J. Use of minicells for bacteriophage-directed polypeptide synthesis. Methods Enzymol. 1979;68:493–503. doi: 10.1016/0076-6879(79)68038-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Stauffer L. T., Ghrist A., Stauffer G. V. The Escherichia coli gcvT gene encoding the T-protein of the glycine cleavage enzyme system. DNA Seq. 1993;3(6):339–346. doi: 10.3109/10425179309020835. [DOI] [PubMed] [Google Scholar]
- Steiert P. S., Stauffer L. T., Stauffer G. V. The lpd gene product functions as the L protein in the Escherichia coli glycine cleavage enzyme system. J Bacteriol. 1990 Oct;172(10):6142–6144. doi: 10.1128/jb.172.10.6142-6144.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Autoregulation by tandem promoters of the Salmonella typhimurium LT2 metJ gene. J Bacteriol. 1986 Mar;165(3):740–745. doi: 10.1128/jb.165.3.740-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wilson R. L., Stauffer L. T., Stauffer G. V. Roles of the GcvA and PurR proteins in negative regulation of the Escherichia coli glycine cleavage enzyme system. J Bacteriol. 1993 Aug;175(16):5129–5134. doi: 10.1128/jb.175.16.5129-5134.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. L., Steiert P. S., Stauffer G. V. Positive regulation of the Escherichia coli glycine cleavage enzyme system. J Bacteriol. 1993 Feb;175(3):902–904. doi: 10.1128/jb.175.3.902-904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]