Abstract
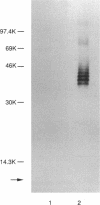
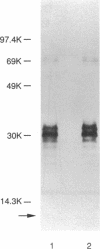
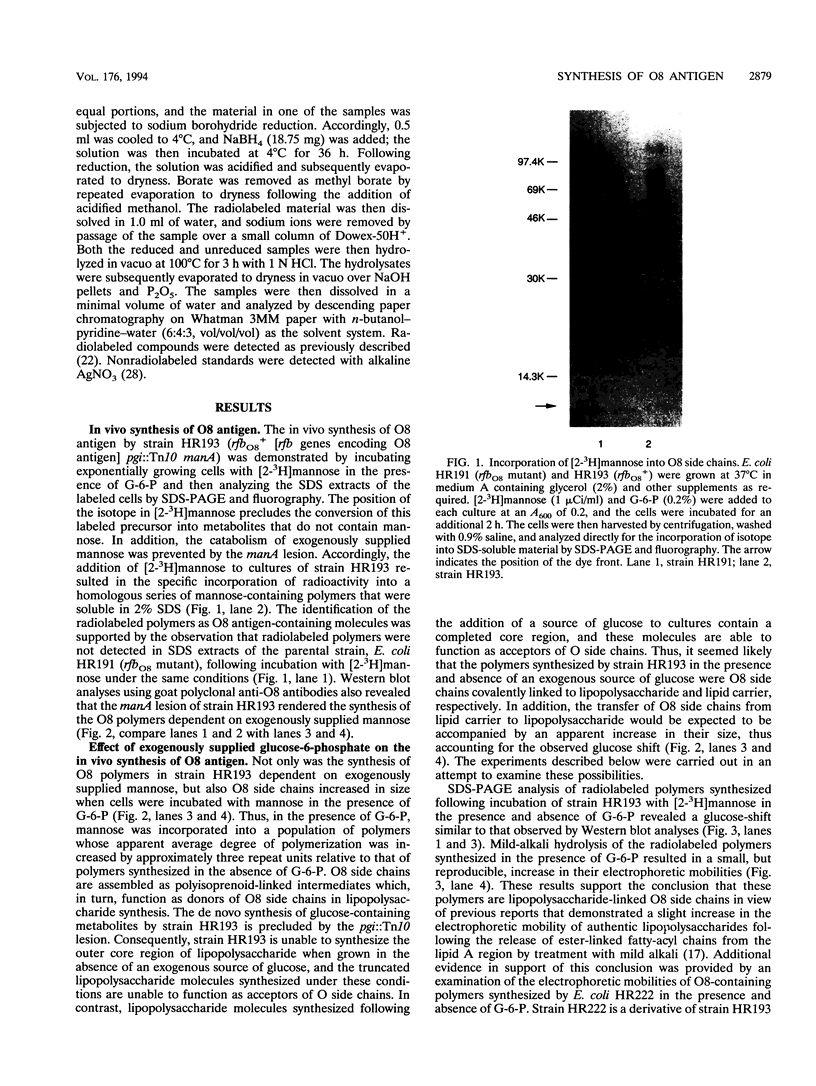
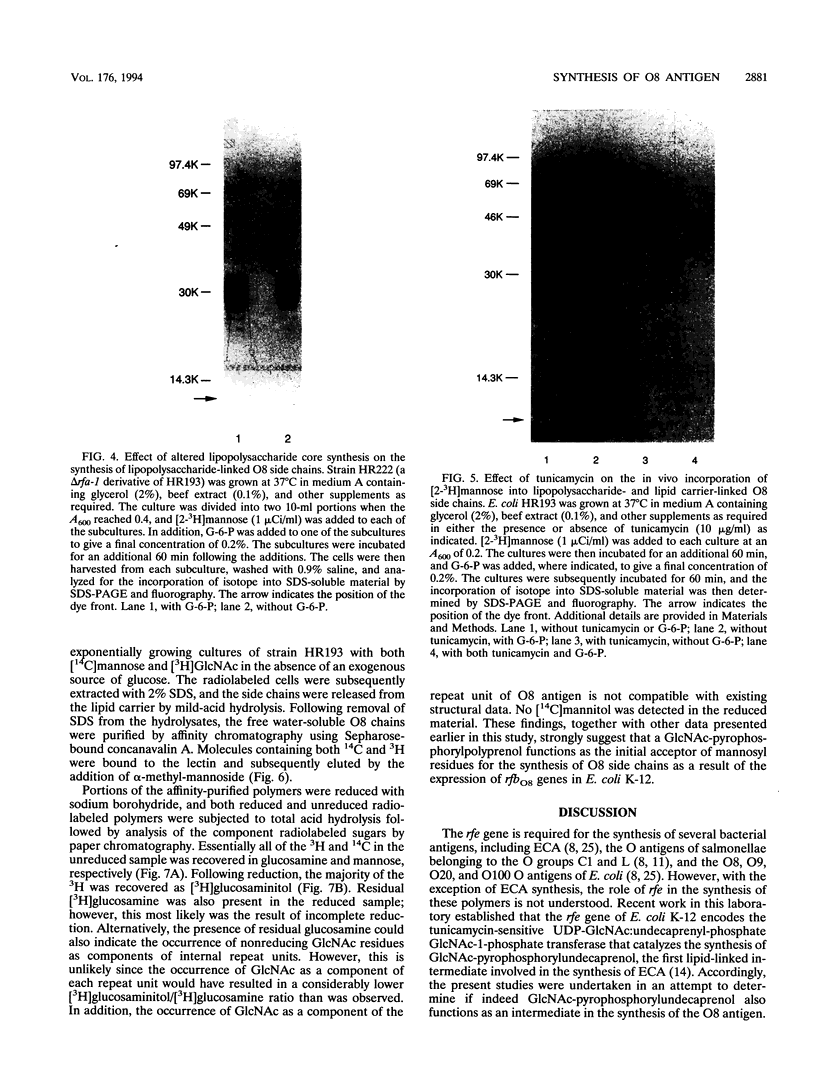
The Escherichia coli O8 antigen is a mannan composed of the trisaccharide repeat unit -->3)-alpha-Man-(1-->2)-alpha-Man-(1-->2)-alpha-Man-(1--> (K. Reske and K. Jann, Eur. J. Biochem. 67:53-56, 1972), and synthesis of the O8 antigen is rfe dependent (G. Schmidt, H. Mayer, and P. H. Mäkelä, J. Bacteriol. 127:755-762, 1976). The rfe gene has recently been identified as encoding a tunicamycin-sensitive UDP-GlcNAc:undecaprenylphosphate GlcNAc-1-phosphate transferase (U. Meier-Dieter, K. Barr, R. Starman, L. Hatch, and P. D. Rick, J. Biol. Chem. 267:746-753, 1992). However, the role of rfe in O8 side chain synthesis is not understood. Thus, the role of the rfe gene in the synthesis of the O8 antigen was investigated in an rfbO8+ (rfb genes encoding O8 antigen) derivative of E. coli K-12 mutant possessing a defective phosphoglucose isomerase (pgi). The in vivo synthesis of O8 side chains was inhibited by the antibiotic tunicamycin. In addition, putative lipid carrier-linked O8 side chains accumulated in vivo when lipopolysaccharide outer core synthesis was precluded by growing cells in the absence of exogenously supplied glucose. The lipid carrier-linked O8 antigen was extracted from cells and treated with mild acid in order to release free O8 side chains. The water-soluble O8 side chains were then purified by affinity chromatography using Sepharose-bound concanavalin A. Characterization of the affinity-purified O8 side chains revealed the occurrence of glucosamine in the reducing terminal position of the polysaccharide chains. The data presented suggest that GlcNAc-pyrophosphorylundecaprenol functions as the acceptor of mannose residues for the in vivo synthesis of O8 side chains in E. coli K-12.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr K., Ward S., Meier-Dieter U., Mayer H., Rick P. D. Characterization of an Escherichia coli rff mutant defective in transfer of N-acetylmannosaminuronic acid (ManNAcA) from UDP-ManNAcA to a lipid-linked intermediate involved in enterobacterial common antigen synthesis. J Bacteriol. 1988 Jan;170(1):228–233. doi: 10.1128/jb.170.1.228-233.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan D. W., Cronan J. E., Jr Genetic characterization of the Escherichia coli cyclopropane fatty acid (cfa) locus and neighboring loci. Mol Gen Genet. 1984;196(2):367–372. doi: 10.1007/BF00328074. [DOI] [PubMed] [Google Scholar]
- Jann K., Goldemann G., Weisgerber C., Wolf-Ullisch C., Kanegasaki S. Biosynthesis of the O9 antigen of Escherichia coli. Initial reaction and overall mechanism. Eur J Biochem. 1982 Sep;127(1):157–164. doi: 10.1111/j.1432-1033.1982.tb06850.x. [DOI] [PubMed] [Google Scholar]
- Jann K., Kanegasaki S., Goldemann G., Mäkelä P. H. On the effect of rfe mutation on the biosynthesis of the 08 and 09 antigens of E. coli. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1185–1191. doi: 10.1016/0006-291x(79)90242-0. [DOI] [PubMed] [Google Scholar]
- Jann K., Pillat M., Weisgerber C., Shibaev V. N., Torgov V. I. Biosynthesis of the O9 antigen of Escherichia coli. Synthetic glycosyldiphosphomoraprenols as probes for requirement of mannose acceptors. Eur J Biochem. 1985 Sep 2;151(2):393–397. doi: 10.1111/j.1432-1033.1985.tb09114.x. [DOI] [PubMed] [Google Scholar]
- Klena J. D., Schnaitman C. A. Function of the rfb gene cluster and the rfe gene in the synthesis of O antigen by Shigella dysenteriae 1. Mol Microbiol. 1993 Jul;9(2):393–402. doi: 10.1111/j.1365-2958.1993.tb01700.x. [DOI] [PubMed] [Google Scholar]
- Kuhn H. M., Meier-Dieter U., Mayer H. ECA, the enterobacterial common antigen. FEMS Microbiol Rev. 1988 Sep;4(3):195–222. doi: 10.1111/j.1574-6968.1988.tb02743.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Duksin D. Biological activities of the two major components of tunicamycin. J Biol Chem. 1979 Jul 25;254(14):6572–6576. [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Dieter U., Barr K., Starman R., Hatch L., Rick P. D. Nucleotide sequence of the Escherichia coli rfe gene involved in the synthesis of enterobacterial common antigen. Molecular cloning of the rfe-rff gene cluster. J Biol Chem. 1992 Jan 15;267(2):746–753. [PubMed] [Google Scholar]
- Meier-Dieter U., Starman R., Barr K., Mayer H., Rick P. D. Biosynthesis of enterobacterial common antigen in Escherichia coli. Biochemical characterization of Tn10 insertion mutants defective in enterobacterial common antigen synthesis. J Biol Chem. 1990 Aug 15;265(23):13490–13497. [PubMed] [Google Scholar]
- Meier U., Mayer H. Genetic location of genes encoding enterobacterial common antigen. J Bacteriol. 1985 Aug;163(2):756–762. doi: 10.1128/jb.163.2.756-762.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. Detoxification of bacterial lipopolysaccharides (endotoxins) by a human neutrophil enzyme. Science. 1986 Oct 10;234(4773):203–205. doi: 10.1126/science.3529396. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Jahkola M., Lüderitz O. A new gene cluster rfe concerned with the biosynthesis of Salmonella lipopolysaccharide. J Gen Microbiol. 1970 Jan;60(1):91–106. doi: 10.1099/00221287-60-1-91. [DOI] [PubMed] [Google Scholar]
- Parker C. T., Kloser A. W., Schnaitman C. A., Stein M. A., Gottesman S., Gibson B. W. Role of the rfaG and rfaP genes in determining the lipopolysaccharide core structure and cell surface properties of Escherichia coli K-12. J Bacteriol. 1992 Apr;174(8):2525–2538. doi: 10.1128/jb.174.8.2525-2538.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P., Jann B., Jann K. The O9 antigen of Escherichia coli. Structure of the polysaccharide chain. Eur J Biochem. 1976 Aug 1;67(1):53–56. doi: 10.1111/j.1432-1033.1976.tb10631.x. [DOI] [PubMed] [Google Scholar]
- Reske K., Jann K. The O8 antigen of Escherichia coli. Structure of the polysaccharide chain. Eur J Biochem. 1972 Dec 4;31(2):320–328. doi: 10.1111/j.1432-1033.1972.tb02536.x. [DOI] [PubMed] [Google Scholar]
- Rick P. D., Mayer H., Neumeyer B. A., Wolski S., Bitter-Suermann D. Biosynthesis of enterobacterial common antigen. J Bacteriol. 1985 May;162(2):494–503. doi: 10.1128/jb.162.2.494-503.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick P. D., Wolski S., Barr K., Ward S., Ramsay-Sharer L. Accumulation of a lipid-linked intermediate involved in enterobacterial common antigen synthesis in Salmonella typhimurium mutants lacking dTDP-glucose pyrophosphorylase. J Bacteriol. 1988 Sep;170(9):4008–4014. doi: 10.1128/jb.170.9.4008-4014.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Bray D., Dankert B. M., Wright A. Direction of chain growth in polysaccharide synthesis. Science. 1967 Dec 22;158(3808):1536–1542. doi: 10.1126/science.158.3808.1536. [DOI] [PubMed] [Google Scholar]
- Rowley D. L., Wolf R. E., Jr Molecular characterization of the Escherichia coli K-12 zwf gene encoding glucose 6-phosphate dehydrogenase. J Bacteriol. 1991 Feb;173(3):968–977. doi: 10.1128/jb.173.3.968-977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Mayer H., Mäkelä P. H. Presence of rfe genes in Escherichia coli: their participation in biosynthesis of O antigen and enterobacterial common antigen. J Bacteriol. 1976 Aug;127(2):755–762. doi: 10.1128/jb.127.2.755-762.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Weisgerber C., Jann B., Jann K. Biosynthesis of the 09 antigen of Escherichia coli. Core structure of rfe mutant as indication of assembly mechanism. Eur J Biochem. 1984 May 2;140(3):553–556. doi: 10.1111/j.1432-1033.1984.tb08137.x. [DOI] [PubMed] [Google Scholar]
- Weisgerber C., Jann K. Glucosyldiphosphoundecaprenol, the mannose acceptor in the synthesis of the O9 antigen of Escherichia coli. Biosynthesis and characterization. Eur J Biochem. 1982 Sep;127(1):165–168. doi: 10.1111/j.1432-1033.1982.tb06851.x. [DOI] [PubMed] [Google Scholar]