Abstract
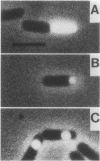
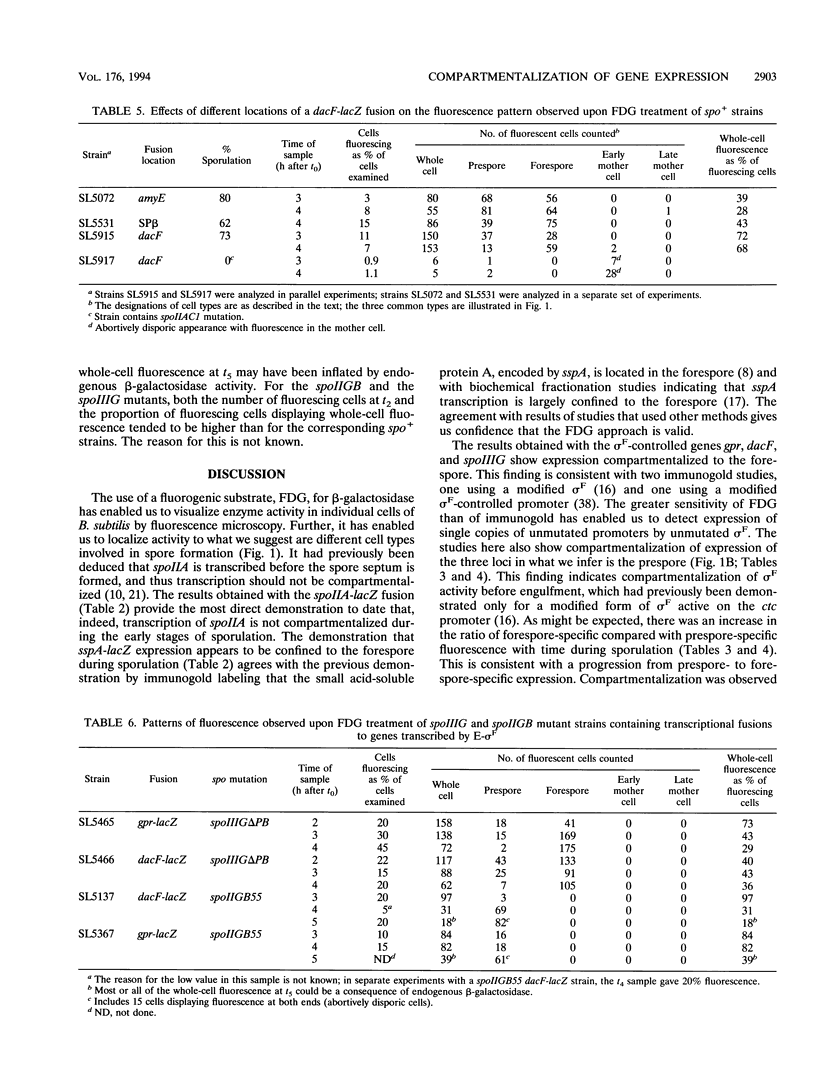
The use of a fluorogenic substrate, 5-octanoylaminofluorescein-di-beta-D-galactopyranoside, for beta-galactosidase has made it possible to visualize enzyme activity in individual cells of sporulating populations of Bacillus subtilis by fluorescence microscopy. lacZ fusions to different sporulation-associated genes have been used to investigate the cell compartmentalization of gene expression during sporulation. A strain with a lacZ fusion to sspA, a gene which is transcribed by E-sigma G at a late stage of sporulation, displayed predominantly compartment-specific fluorescence. Expression of the early-expressed spoIIA locus, which includes the structural gene for sigma F, was seen not to be compartmentalized. Populations of strains with lacZ fusions to gpr and dacF, genes which are transcribed by E-sigma F at intermediate stages of sporulation, included some organisms showing uncompartmentalized fluorescence and others showing compartment-specific fluorescence; the proportion showing compartment-specific fluorescence increased in samples taken later in sporulation. Several possible explanations of the results obtained with gpr and dacF are considered. A plausible interpretation is that sigma F activity is initially not compartmentalized and becomes compartmentalized as sporulation progresses. The progression to compartmentalization does not require the activities of the sporulation-specific factor sigma E or sigma G but may require some product of sigma F activity.
Full text
PDF

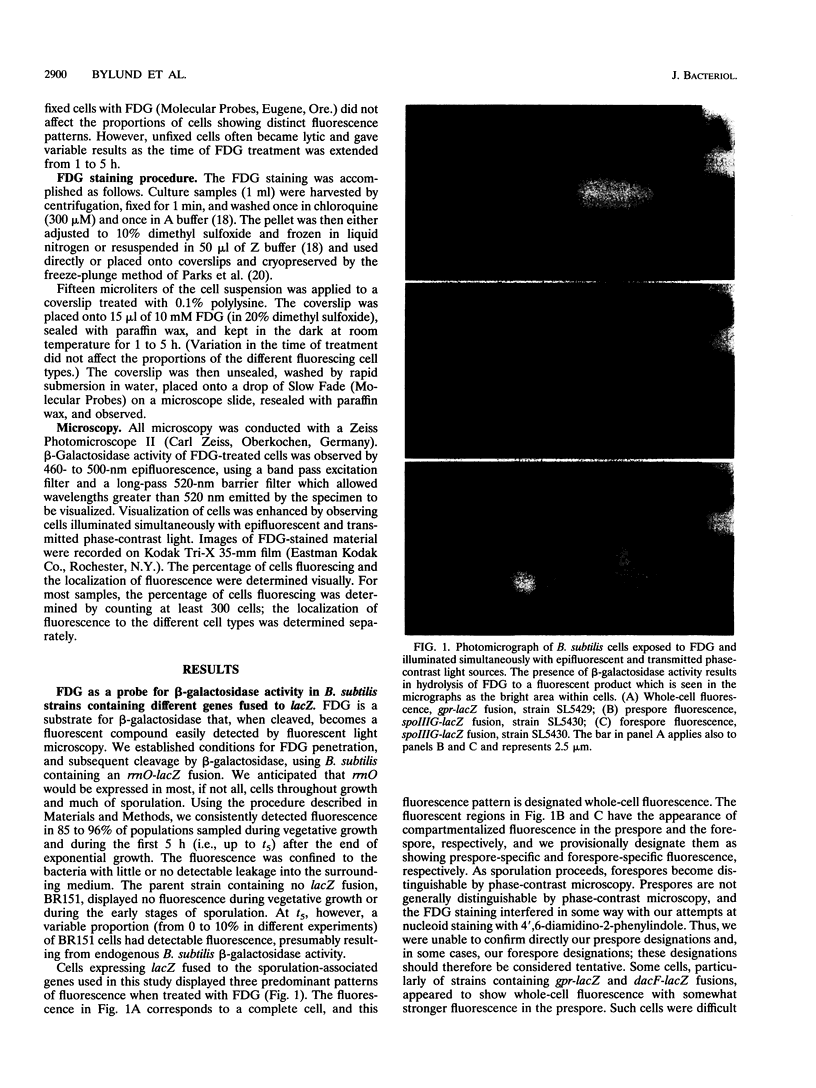


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Driks A., Losick R. Compartmentalized expression of a gene under the control of sporulation transcription factor sigma E in Bacillus subtilis. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9934–9938. doi: 10.1073/pnas.88.22.9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton M. W., Ellar D. J. Protein synthesis and breakdown in the mother-cell and forespore compartments during spore morphogenesis in Bacillus megaterium. Biochem J. 1974 Nov;144(2):327–337. doi: 10.1042/bj1440327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993 Mar;57(1):1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington J., Vogt C. H. Isolation and characterization of mutations in the gene encoding an endogenous Bacillus subtilis beta-galactosidase and its regulator. J Bacteriol. 1990 Jan;172(1):488–490. doi: 10.1128/jb.172.1.488-490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi S. C., MacAlister T. J., Setlow B., Setlow P. Immunoelectron microscopic localization of small, acid-soluble spore proteins in sporulating cells of Bacillus subtilis. J Bacteriol. 1988 Dec;170(12):5963–5967. doi: 10.1128/jb.170.12.5963-5967.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Ramaley R., Freese E. Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis. J Bacteriol. 1977 Oct;132(1):282–293. doi: 10.1128/jb.132.1.282-293.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamhoseinian A., Piggot P. J. Timing of spoII gene expression relative to septum formation during sporulation of Bacillus subtilis. J Bacteriol. 1989 Oct;171(10):5747–5749. doi: 10.1128/jb.171.10.5747-5749.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Piggot P. J. Septal membrane fusion--a pivotal event in bacterial spore formation? Mol Microbiol. 1992 Sep;6(18):2565–2571. doi: 10.1111/j.1365-2958.1992.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Hranueli D., Piggot P. J., Mandelstam J. Statistical estimate of the total number of operons specific for Bacillus subtilis sporulation. J Bacteriol. 1974 Sep;119(3):684–690. doi: 10.1128/jb.119.3.684-690.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing N., Errington J. Genetic regulation of morphogenesis in Bacillus subtilis: roles of sigma E and sigma F in prespore engulfment. J Bacteriol. 1991 May;173(10):3159–3169. doi: 10.1128/jb.173.10.3159-3169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmazyn-Campelli C., Bonamy C., Savelli B., Stragier P. Tandem genes encoding sigma-factors for consecutive steps of development in Bacillus subtilis. Genes Dev. 1989 Feb;3(2):150–157. doi: 10.1101/gad.3.2.150. [DOI] [PubMed] [Google Scholar]
- Losick R., Stragier P. Crisscross regulation of cell-type-specific gene expression during development in B. subtilis. Nature. 1992 Feb 13;355(6361):601–604. doi: 10.1038/355601a0. [DOI] [PubMed] [Google Scholar]
- Margolis P., Driks A., Losick R. Establishment of cell type by compartmentalized activation of a transcription factor. Science. 1991 Oct 25;254(5031):562–565. doi: 10.1126/science.1948031. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Hackett R. H., Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988 Jan;170(1):239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir R., Yisraeli Y., Lamed R., Sahar E. Flow cytometry sorting of viable bacteria and yeasts according to beta-galactosidase activity. Appl Environ Microbiol. 1990 Dec;56(12):3861–3866. doi: 10.1128/aem.56.12.3861-3866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. C., Dicker D. T., Conger A. D., Daneo-Moore L., Higgins M. L. Effect of chromosomal breaks induced by x-irradiation on the number of mesosomes and the cytoplasmic organization of Streptococcus faecalis. J Mol Biol. 1981 Mar 15;146(4):413–431. doi: 10.1016/0022-2836(81)90040-1. [DOI] [PubMed] [Google Scholar]
- Partridge S. R., Errington J. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol Microbiol. 1993 May;8(5):945–955. doi: 10.1111/j.1365-2958.1993.tb01639.x. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J. Mapping of asporogenous mutations of Bacillus subtilis: a minimum estimate of the number of sporeulation operons. J Bacteriol. 1973 Jun;114(3):1241–1253. doi: 10.1128/jb.114.3.1241-1253.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVEL H. R., LURIA S. E., ROTMAN B. Biosynthesis of B-D-galactosidase controlled by phage-carried genes. I. Induced beta-D-galactosidase biosynthesis after transduction of gene z-plus by phage. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1956–1967. doi: 10.1073/pnas.47.12.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTMAN B. Measurement of activity of single molecules of beta-D-galactosidase. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1981–1991. doi: 10.1073/pnas.47.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaley R. F., Burden L. Replacement sporulation of Bacillus subtilis 168 in a chemically defined medium. J Bacteriol. 1970 Jan;101(1):1–8. doi: 10.1128/jb.101.1.1-8.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Marie F., Roederer M., Sager B., Herzenberg L. A., Kaiser D. Beta-galactosidase activity in single differentiating bacterial cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8194–8198. doi: 10.1073/pnas.90.17.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Margolis P., Duncan L., Coppolecchia R., Moran C. P., Jr, Losick R. Control of developmental transcription factor sigma F by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9221–9225. doi: 10.1073/pnas.87.23.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- Shimotsu H., Henner D. J. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene. 1986;43(1-2):85–94. doi: 10.1016/0378-1119(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K., Youngman P. Evidence that the spoIIM gene of Bacillus subtilis is transcribed by RNA polymerase associated with sigma E. J Bacteriol. 1993 Jun;175(11):3618–3627. doi: 10.1128/jb.175.11.3618-3627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier P., Bouvier J., Bonamy C., Szulmajster J. A developmental gene product of Bacillus subtilis homologous to the sigma factor of Escherichia coli. Nature. 1984 Nov 22;312(5992):376–378. doi: 10.1038/312376a0. [DOI] [PubMed] [Google Scholar]
- Sun D. X., Stragier P., Setlow P. Identification of a new sigma-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 1989 Feb;3(2):141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- Sun D., Fajardo-Cavazos P., Sussman M. D., Tovar-Rojo F., Cabrera-Martinez R. M., Setlow P. Effect of chromosome location of Bacillus subtilis forespore genes on their spo gene dependence and transcription by E sigma F: identification of features of good E sigma F-dependent promoters. J Bacteriol. 1991 Dec;173(24):7867–7874. doi: 10.1128/jb.173.24.7867-7874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. D., Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis gpr gene, which codes for the protease that initiates degradation of small, acid-soluble proteins during spore germination. J Bacteriol. 1991 Jan;173(1):291–300. doi: 10.1128/jb.173.1.291-300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrup K. D., Bailey J. E. A single-cell assay of beta-galactosidase activity in Saccharomyces cerevisiae. Cytometry. 1988 Jul;9(4):394–404. doi: 10.1002/cyto.990090418. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Howard M. G., Piggot P. J. Regulation of transcription of the Bacillus subtilis spoIIA locus. J Bacteriol. 1989 Feb;171(2):692–698. doi: 10.1128/jb.171.2.692-698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. J., Piggot P. J., Tatti K. M., Moran C. P., Jr Transcription of the Bacillus subtilis spoIIA locus. Gene. 1991 May 15;101(1):113–116. doi: 10.1016/0378-1119(91)90231-y. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Schuch R., Piggot P. J. Characterization of a Bacillus subtilis sporulation operon that includes genes for an RNA polymerase sigma factor and for a putative DD-carboxypeptidase. J Bacteriol. 1992 Aug;174(15):4885–4892. doi: 10.1128/jb.174.15.4885-4892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin M. D. Structure and function in a Bacillus subtilis sporulation-specific sigma factor: molecular nature of mutations in spoIIAC. J Gen Microbiol. 1987 Mar;133(3):475–481. doi: 10.1099/00221287-133-3-475. [DOI] [PubMed] [Google Scholar]
- Zuber P., Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lencastre H., Piggot P. J. Identification of different sites of expression for spo loci by transformation of Bacillus subtilis. J Gen Microbiol. 1979 Oct;114(2):377–389. doi: 10.1099/00221287-114-2-377. [DOI] [PubMed] [Google Scholar]