Abstract
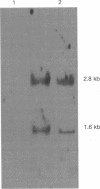
Carnitine dehydratase from Escherichia coli O44 K74 is an inducible enzyme detectable in cells grown anaerobically in the presence of L-(-)-carnitine or crotonobetaine. The purified enzyme catalyzes the dehydration of L-(-)-carnitine to crotonobetaine (H. Jung, K. Jung, and H.-P. Kleber, Biochim. Biophys. Acta 1003:270-276, 1989). The caiB gene, encoding carnitine dehydratase, was isolated by oligonucleotide screening from a genomic library of E. coli O44 K74. The caiB gene is 1,215 bp long, and it encodes a protein of 405 amino acids with a predicted M(r) of 45,074. The identity of the gene product was first assessed by its comigration in sodium dodecyl sulfate-polyacrylamide gels with the purified enzyme after overexpression in the pT7 system and by its enzymatic activity. Moreover, the N-terminal amino acid sequence of the purified protein was found to be identical to that predicted from the gene sequence. Northern (RNA) analysis showed that caiB is likely to be cotranscribed with at least one other gene. This other gene could be the gene encoding a 47-kDa protein, which was overexpressed upstream of caiB.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardonnet N., Blanco C. 'uidA-antibiotic-resistance cassettes for insertion mutagenesis, gene fusions and genetic constructions. FEMS Microbiol Lett. 1992 Jun 15;72(3):243–247. doi: 10.1016/0378-1097(92)90469-5. [DOI] [PubMed] [Google Scholar]
- Bremer J. Carnitine--metabolism and functions. Physiol Rev. 1983 Oct;63(4):1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Jung H., Jung K., Kleber H. P. Purification and properties of carnitine dehydratase from Escherichia coli--a new enzyme of carnitine metabolization. Biochim Biophys Acta. 1989 Jun 28;1003(3):270–276. doi: 10.1016/0005-2760(89)90232-4. [DOI] [PubMed] [Google Scholar]
- Jung K., Jung H., Kleber H. P. Regulation of L-carnitine metabolism in Escherichia coli. J Basic Microbiol. 1987;27(3):131–137. doi: 10.1002/jobm.3620270303. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mallonee D. H., White W. B., Hylemon P. B. Cloning and sequencing of a bile acid-inducible operon from Eubacterium sp. strain VPI 12708. J Bacteriol. 1990 Dec;172(12):7011–7019. doi: 10.1128/jb.172.12.7011-7019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seim H., Ezold R., Kleber H. P., Strack E. Stoffwechsel des L-Carnitins bei Enterobakterien. Z Allg Mikrobiol. 1980;20(9):591–594. doi: 10.1002/jobm.3630200909. [DOI] [PubMed] [Google Scholar]
- Seim H., Kleber H. P., Strack E. Reduktion von L-Carnitin zu gamma-Butyrobetain durch Escherichia coli. Z Allg Mikrobiol. 1979;19(10):753–758. doi: 10.1002/jobm.3630191011. [DOI] [PubMed] [Google Scholar]
- Seim H., Löster H., Claus R., Kleber H. P., Strack E. Stimulation of the anaerobic growth of Salmonella typhimurium by reduction of L-carnitine, carnitine derivatives and structure-related trimethylammonium compounds. Arch Microbiol. 1982 Jul;132(1):91–95. doi: 10.1007/BF00690825. [DOI] [PubMed] [Google Scholar]
- Seim H., Löster H., Kleber H. P. Reduktiver Stoffwechsel des L-Carnitins und strukturverwandter Trimethylammoniumverbindungen in Escherichia coli. Acta Biol Med Ger. 1982;41(11):1009–1018. [PubMed] [Google Scholar]
- Shimotsu H., Kuroda M. I., Yanofsky C., Henner D. J. Novel form of transcription attenuation regulates expression the Bacillus subtilis tryptophan operon. J Bacteriol. 1986 May;166(2):461–471. doi: 10.1128/jb.166.2.461-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typlt H., Claus R., Nitzsche K. Influence of carnitine on the growth and productivity of murine hybridoma cells. J Biotechnol. 1991 Apr;18(1-2):173–175. doi: 10.1016/0168-1656(91)90245-q. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. F., Mandrand-Berthelot M. A. Genetic and physiological characterization of new Escherichia coli mutants impaired in hydrogenase activity. Biochimie. 1986 Jan;68(1):167–179. doi: 10.1016/s0300-9084(86)81081-1. [DOI] [PubMed] [Google Scholar]