Abstract
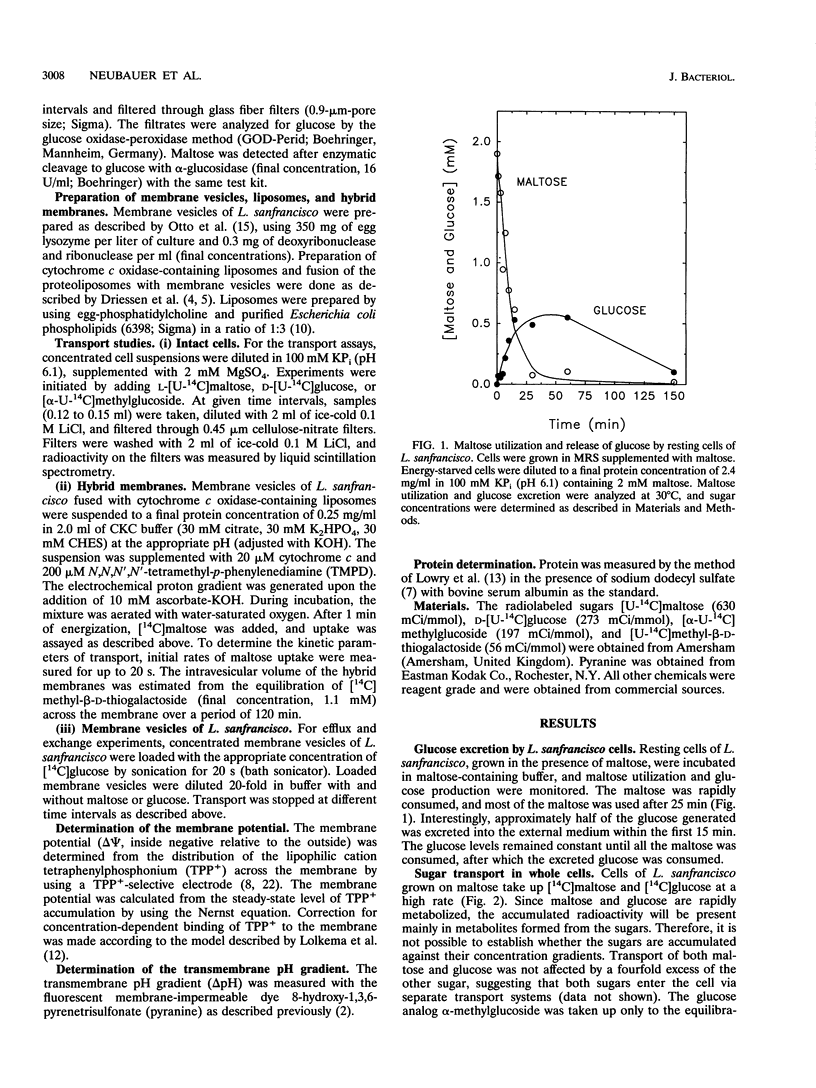
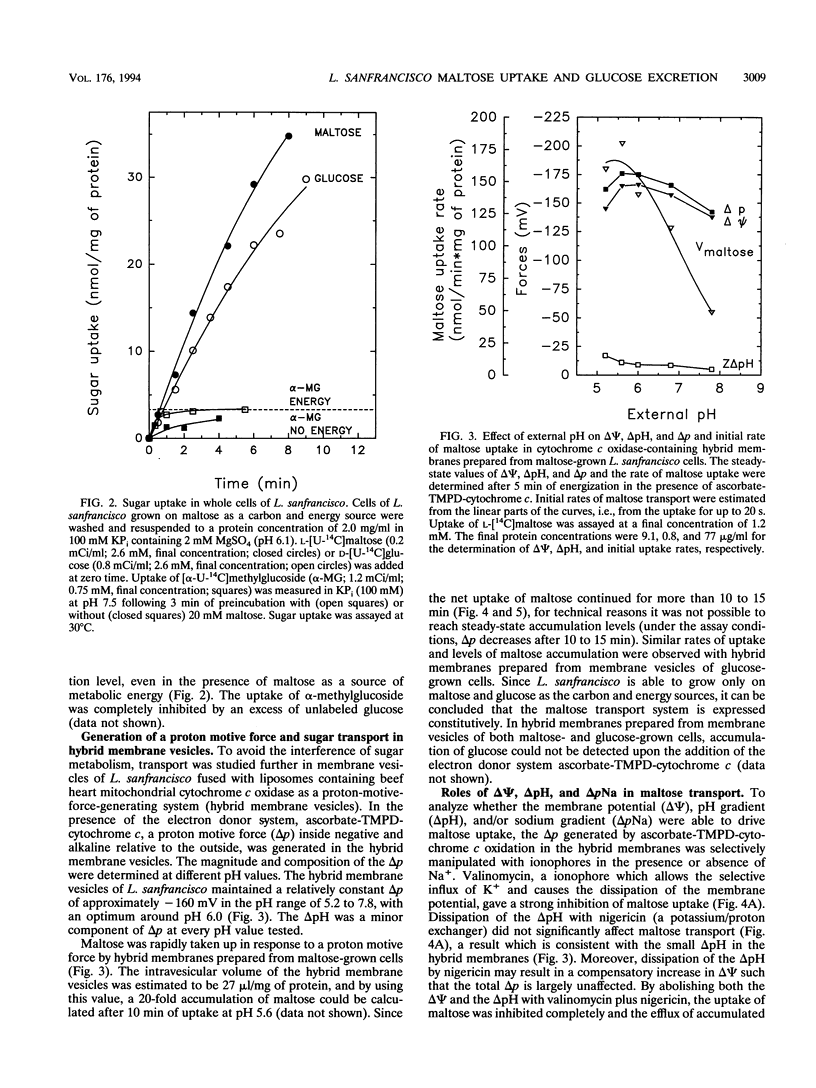
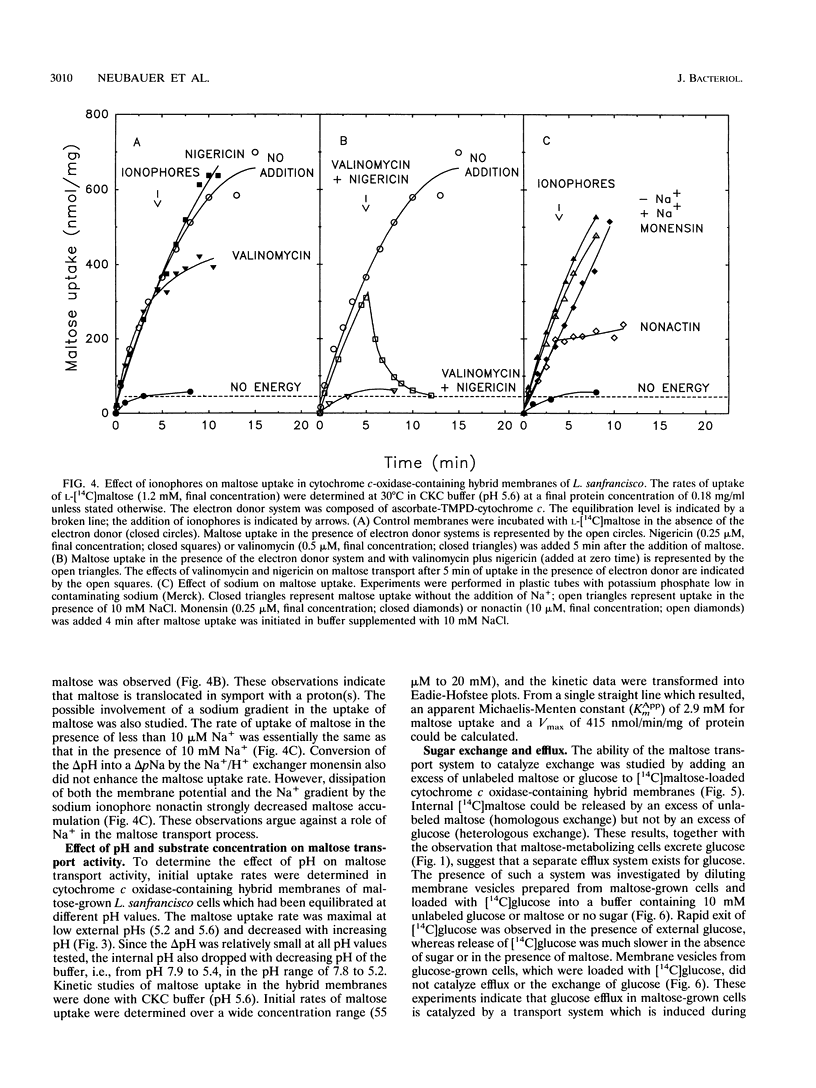
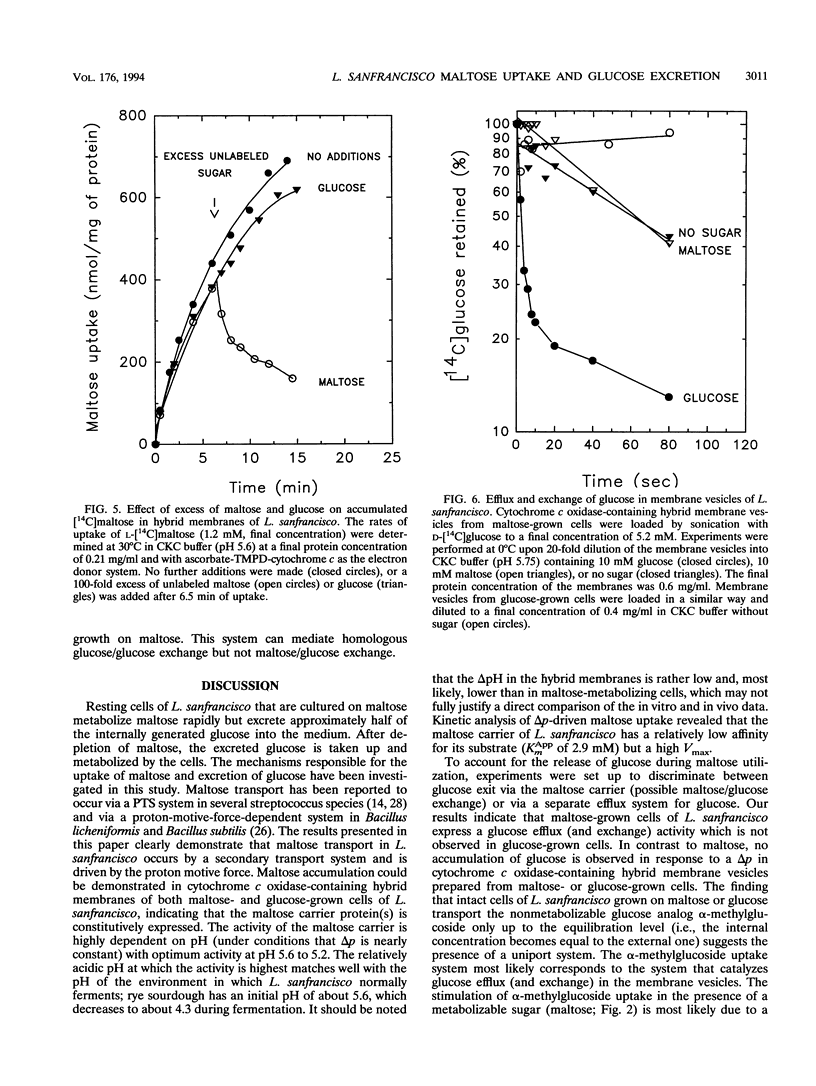
Lactobacillus sanfrancisco LTH 2581 can use only glucose and maltose as sources of metabolic energy. In maltose-metabolizing cells of L. sanfrancisco, approximately half of the internally generated glucose appears in the medium. The mechanisms of maltose (and glucose) uptake and glucose excretion have been investigated in cells and in membrane vesicles of L. sanfrancisco in which beef heart cytochrome c oxidase had been incorporated as a proton-motive-force-generating system. In the presence of ascorbate, N,N,N',N'-tetramethyl-p-phenylenediamine (TMPD), and cytochrome c, the hybrid membranes facilitated maltose uptake against a concentration gradient, but accumulation of glucose could not be detected. Similarly, in intact cells of L. sanfrancisco, the nonmetabolizable glucose analog alpha-methylglucoside was taken up only to the equilibration level. Selective dissipation of the components of the proton and sodium motive force in the hybrid membranes indicated that maltose is transported by a proton symport mechanism. Internal [14C]maltose could be chased with external unlabeled maltose (homologous exchange), but heterologous maltose/glucose exchange could not be detected. Membrane vesicles of L. sanfrancisco also catalyzed glucose efflux and homologous glucose exchange. These activities could not be detected in membrane vesicles of glucose-grown cells. The results indicate that maltose-grown cells of L. sanfrancisco express a maltose-H+ symport and glucose uniport system. When maltose is the substrate, the formation of intracellular glucose can be more rapid than the subsequent metabolism, which leads to excretion of glucose via the uniport system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Damiano E., Bassilana M., Rigaud J. L., Leblanc G. Use of the pH sensitive fluorescence probe pyranine to monitor internal pH changes in Escherichia coli membrane vesicles. FEBS Lett. 1984 Jan 23;166(1):120–124. doi: 10.1016/0014-5793(84)80056-3. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., Poolman B., Kiewiet R., Konings W. Arginine transport in Streptococcus lactis is catalyzed by a cationic exchanger. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6093–6097. doi: 10.1073/pnas.84.17.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Functional incorporation of beef-heart cytochrome c oxidase into membranes of Streptococcus cremoris. Eur J Biochem. 1986 Feb 3;154(3):617–624. doi: 10.1111/j.1432-1033.1986.tb09443.x. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Incorporation of beef heart cytochrome c oxidase as a proton-motive force-generating mechanism in bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7555–7559. doi: 10.1073/pnas.82.22.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Foucaud C., Poolman B. Lactose transport system of Streptococcus thermophilus. Functional reconstitution of the protein and characterization of the kinetic mechanism of transport. J Biol Chem. 1992 Nov 5;267(31):22087–22094. [PubMed] [Google Scholar]
- Kagawa Y., Kandrach A., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXVI. Specificity of phospholipids required for energy transfer reactions. J Biol Chem. 1973 Jan 25;248(2):676–684. [PubMed] [Google Scholar]
- Kline L., Sugihara T. F. Microorganisms of the San Francisco sour dough bread process. II. Isolation and characterization of undescribed bacterial species responsible for the souring activity. Appl Microbiol. 1971 Mar;21(3):459–465. doi: 10.1128/am.21.3.459-465.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Transport and phosphorylation of disaccharides by the ruminal bacterium Streptococcus bovis. Appl Environ Microbiol. 1987 Oct;53(10):2388–2393. doi: 10.1128/aem.53.10.2388-2393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol. 1987 Dec;169(12):5597–5604. doi: 10.1128/jb.169.12.5597-5604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Konings W. N. Secondary solute transport in bacteria. Biochim Biophys Acta. 1993 Nov 2;1183(1):5–39. doi: 10.1016/0005-2728(93)90003-x. [DOI] [PubMed] [Google Scholar]
- Poolman B. Precursor/product antiport in bacteria. Mol Microbiol. 1990 Oct;4(10):1629–1636. doi: 10.1111/j.1365-2958.1990.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Poolman B., Royer T. J., Mainzer S. E., Schmidt B. F. Carbohydrate utilization in Streptococcus thermophilus: characterization of the genes for aldose 1-epimerase (mutarotase) and UDPglucose 4-epimerase. J Bacteriol. 1990 Jul;172(7):4037–4047. doi: 10.1128/jb.172.7.4037-4047.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Royer T. J., Mainzer S. E., Schmidt B. F. Lactose transport system of Streptococcus thermophilus: a hybrid protein with homology to the melibiose carrier and enzyme III of phosphoenolpyruvate-dependent phosphotransferase systems. J Bacteriol. 1989 Jan;171(1):244–253. doi: 10.1128/jb.171.1.244-253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Spicher G., Schröder R. Die Mikroflora des Sauerteiges. IV. Mitteilung: Untersuchungen über die Art der in "Reinzuchtsauern" anzutreffenden stäbchenförmigen Milchsäurebakterien (Genus Lactobacillus Beijerinck). Z Lebensm Unters Forsch. 1978 Nov 28;167(5):342–354. doi: 10.1007/BF01415931. [DOI] [PubMed] [Google Scholar]
- Würsch P., Koellreutter B. Maltotriitol inhibition of maltose metabolism in Streptococcus mutans via maltose transport, amylomaltase and phospho-alpha-glucosidase activities. Caries Res. 1985;19(5):439–449. doi: 10.1159/000260879. [DOI] [PubMed] [Google Scholar]


