Abstract
Expression of the glucitol dehydrogenase gene (gutB) is suggested to be regulated both positively and negatively in Bacillus subtilis. A mutation in the gutR locus results in the constitutive expression of gutB. The exact nature of this mutation and the function of gutR are still unknown. Cloning and characterization of gutR indicated that this gene is located immediately upstream of gutB and is transcribed in the opposite direction relative to gutB. GutR is suggested to be a 95-kDa protein with a putative helix-turn-helix motif and a nucleotide binding domain at the N-terminal region. At the C-terminal region, a short sequence of GutR shows homology with two proteins, Cyc8 (glucose repression mediator protein) and GsiA (glucose starvation-inducible protein), known to be directly or indirectly involved in catabolite repression. Part of the C-terminal conserved sequence from these proteins shows all the features observed in the tetratricopeptide motif found in many eucaryotic proteins. To study the functional role of gutR, chromosomal gutR was insertionally inactivated. A total loss of glucitol inducibility was observed. Reintroduction of a functional gutR to the GutR-deficient strain through integration at the amyE locus restores the inducibility. Therefore, GutR serves as a regulatory factor to modulate glucitol induction. The nature of the gutR1 mutation was also determined. A single amino acid change (serine-289 to arginine-289) near the putative nucleotide binding motif B in GutR is responsible for the observed phenotype. Possible models for the action of GutR are discussed.
Full text
PDF
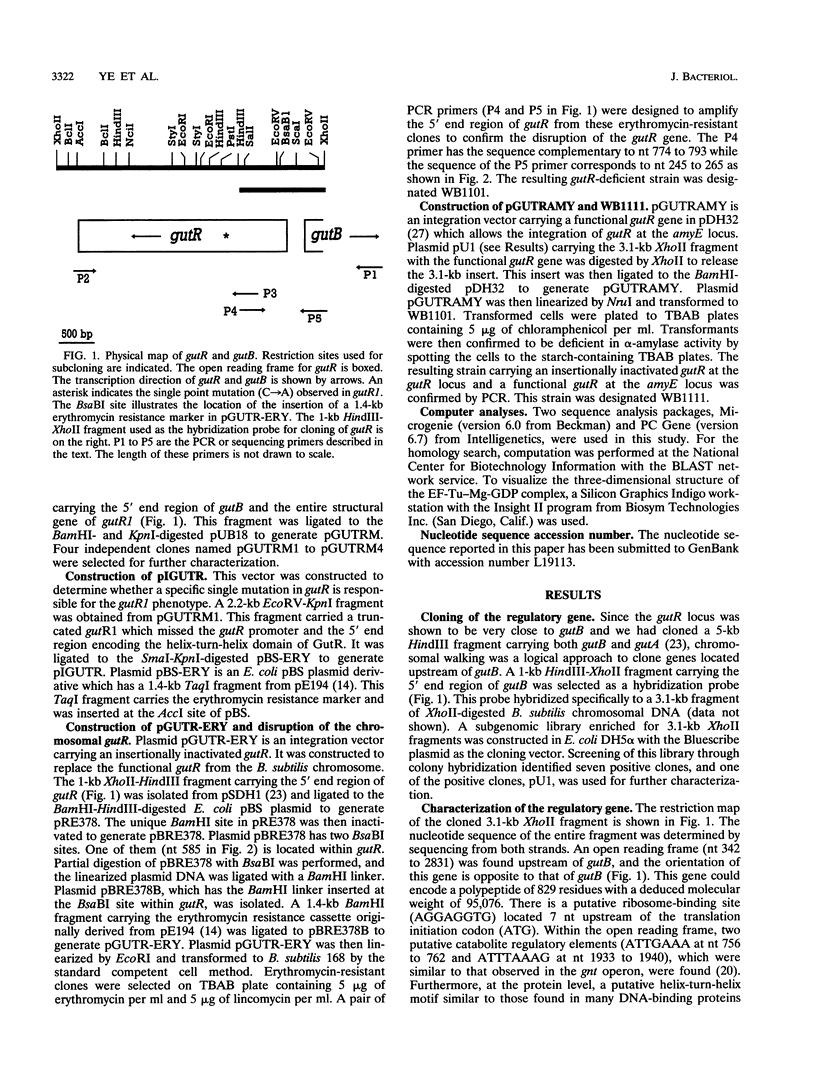




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. Z., Chael M. L., O'Halloran T. V. Allosteric underwinding of DNA is a critical step in positive control of transcription by Hg-MerR. Nature. 1992 Jan 2;355(6355):87–89. doi: 10.1038/355087a0. [DOI] [PubMed] [Google Scholar]
- Austin S., Dixon R. The prokaryotic enhancer binding protein NTRC has an ATPase activity which is phosphorylation and DNA dependent. EMBO J. 1992 Jun;11(6):2219–2228. doi: 10.1002/j.1460-2075.1992.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymerich S., Steinmetz M. Specificity determinants and structural features in the RNA target of the bacterial antiterminator proteins of the BglG/SacY family. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10410–10414. doi: 10.1073/pnas.89.21.10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalumeau H., Delobbe A., Gay P. Biochemical and genetic study of D-glucitol transport and catabolism in Bacillus subtilis. J Bacteriol. 1978 Jun;134(3):920–928. doi: 10.1128/jb.134.3.920-928.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. L., Shuman H. A., Nikaido H. Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2360–2364. doi: 10.1073/pnas.89.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Débarbouillé M., Martin-Verstraete I., Klier A., Rapoport G. The transcriptional regulator LevR of Bacillus subtilis has domains homologous to both sigma 54- and phosphotransferase system-dependent regulators. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2212–2216. doi: 10.1073/pnas.88.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz B., O'Halloran T. V. DNA distortion accompanies transcriptional activation by the metal-responsive gene-regulatory protein MerR. Biochemistry. 1990 May 22;29(20):4747–4751. doi: 10.1021/bi00472a001. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Fujita T. The gluconate operon gnt of Bacillus subtilis encodes its own transcriptional negative regulator. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4524–4528. doi: 10.1073/pnas.84.13.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P., Chalumeau H., Steinmetz M. Chromosomal localization of gut, fruC, and pfk mutations affecting genes involved in Bacillus subtilis D-glucitol catabolism. J Bacteriol. 1983 Mar;153(3):1133–1137. doi: 10.1128/jb.153.3.1133-1137.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner D., Degenkolb J., Ripperger J. A., Allmansberger R., Hillen W. Regulation of the Bacillus subtilis W23 xylose utilization operon: interaction of the Xyl repressor with the xyl operator and the inducer xylose. Mol Gen Genet. 1992 Apr;232(3):415–422. doi: 10.1007/BF00266245. [DOI] [PubMed] [Google Scholar]
- Gärtner D., Geissendörfer M., Hillen W. Expression of the Bacillus subtilis xyl operon is repressed at the level of transcription and is induced by xylose. J Bacteriol. 1988 Jul;170(7):3102–3109. doi: 10.1128/jb.170.7.3102-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Sanchez-Anzaldo F. J., Fukuda R., Doi R. H., Meares C. F. Zinc is associated with the beta subunit of DNA-dependent RNA polymerase of Bacillus subtilis. Biochemistry. 1977 Jun 28;16(13):2880–2884. doi: 10.1021/bi00632a012. [DOI] [PubMed] [Google Scholar]
- Holmberg C., Rutberg L. An inverted repeat preceding the Bacillus subtilis glpD gene is a conditional terminator of transcription. Mol Microbiol. 1992 Oct;6(20):2931–2938. doi: 10.1111/j.1365-2958.1992.tb01752.x. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klier A. F., Rapoport G. Genetics and regulation of carbohydrate catabolism in Bacillus. Annu Rev Microbiol. 1988;42:65–95. doi: 10.1146/annurev.mi.42.100188.000433. [DOI] [PubMed] [Google Scholar]
- Klier A., Msadek T., Rapoport G. Positive regulation in the gram-positive bacterium: Bacillus subtilis. Annu Rev Microbiol. 1992;46:429–459. doi: 10.1146/annurev.mi.46.100192.002241. [DOI] [PubMed] [Google Scholar]
- Li M., Wong S. L. Cloning and characterization of the groESL operon from Bacillus subtilis. J Bacteriol. 1992 Jun;174(12):3981–3992. doi: 10.1128/jb.174.12.3981-3992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Verstraete I., Débarbouillé M., Klier A., Rapoport G. Mutagenesis of the Bacillus subtilis "-12, -24" promoter of the levanase operon and evidence for the existence of an upstream activating sequence. J Mol Biol. 1992 Jul 5;226(1):85–99. doi: 10.1016/0022-2836(92)90126-5. [DOI] [PubMed] [Google Scholar]
- Miwa Y., Fujita Y. Determination of the cis sequence involved in catabolite repression of the Bacillus subtilis gnt operon; implication of a consensus sequence in catabolite repression in the genus Bacillus. Nucleic Acids Res. 1990 Dec 11;18(23):7049–7053. doi: 10.1093/nar/18.23.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. P., Bukusoglu G., Sonenshein A. L. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J Bacteriol. 1992 Jul;174(13):4361–4373. doi: 10.1128/jb.174.13.4361-4373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. P., Sonenshein A. L. Role of the Bacillus subtilis gsiA gene in regulation of early sporulation gene expression. J Bacteriol. 1992 Jul;174(13):4374–4383. doi: 10.1128/jb.174.13.4374-4383.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K., Ye R., Wu X. C., Wong S. L. Sorbitol dehydrogenase from Bacillus subtilis. Purification, characterization, and gene cloning. J Biol Chem. 1992 Dec 15;267(35):24989–24994. [PubMed] [Google Scholar]
- O'Halloran T. V., Frantz B., Shin M. K., Ralston D. M., Wright J. G. The MerR heavy metal receptor mediates positive activation in a topologically novel transcription complex. Cell. 1989 Jan 13;56(1):119–129. doi: 10.1016/0092-8674(89)90990-2. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Parkhill J., Brown N. L. Site-specific insertion and deletion mutants in the mer promoter-operator region of Tn501; the nineteen base-pair spacer is essential for normal induction of the promoter by MerR. Nucleic Acids Res. 1990 Sep 11;18(17):5157–5162. doi: 10.1093/nar/18.17.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popham D. L., Szeto D., Keener J., Kustu S. Function of a bacterial activator protein that binds to transcriptional enhancers. Science. 1989 Feb 3;243(4891):629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- Shimotsu H., Henner D. J. Modulation of Bacillus subtilis levansucrase gene expression by sucrose and regulation of the steady-state mRNA level by sacU and sacQ genes. J Bacteriol. 1986 Oct;168(1):380–388. doi: 10.1128/jb.168.1.380-388.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Boguski M. S., Goebl M., Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990 Jan 26;60(2):307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Le Coq D., Aymerich S., Gonzy-Tréboul G., Gay P. The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol Gen Genet. 1985;200(2):220–228. doi: 10.1007/BF00425427. [DOI] [PubMed] [Google Scholar]
- Su W., Porter S., Kustu S., Echols H. DNA-looping and enhancer activity: association between DNA-bound NtrC activator and RNA polymerase at the bacterial glnA promoter. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5504–5508. doi: 10.1073/pnas.87.14.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbly R. J. Cloning and characterization of the CYC8 gene mediating glucose repression in yeast. Gene. 1988 Dec 15;73(1):97–111. doi: 10.1016/0378-1119(88)90316-2. [DOI] [PubMed] [Google Scholar]
- Wang L. F., Doi R. H. Promoter switching during development and the termination site of the sigma 43 operon of Bacillus subtilis. Mol Gen Genet. 1987 Apr;207(1):114–119. doi: 10.1007/BF00331498. [DOI] [PubMed] [Google Scholar]
- Wong S. L., Wang L. F., Doi R. H. Cloning and nucleotide sequence of senN, a novel 'Bacillus natto' (B. subtilis) gene that regulates expression of extracellular protein genes. J Gen Microbiol. 1988 Dec;134(12):3269–3276. doi: 10.1099/00221287-134-12-3269. [DOI] [PubMed] [Google Scholar]
- Wu X. C., Nathoo S., Pang A. S., Carne T., Wong S. L. Cloning, genetic organization, and characterization of a structural gene encoding bacillopeptidase F from Bacillus subtilis. J Biol Chem. 1990 Apr 25;265(12):6845–6850. [PubMed] [Google Scholar]
- Ye R., Wong S. L. Transcriptional regulation of the Bacillus subtilis glucitol dehydrogenase gene. J Bacteriol. 1994 Jun;176(11):3314–3320. doi: 10.1128/jb.176.11.3314-3320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Cour T. F., Nyborg J., Thirup S., Clark B. F. Structural details of the binding of guanosine diphosphate to elongation factor Tu from E. coli as studied by X-ray crystallography. EMBO J. 1985 Sep;4(9):2385–2388. doi: 10.1002/j.1460-2075.1985.tb03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]