Abstract
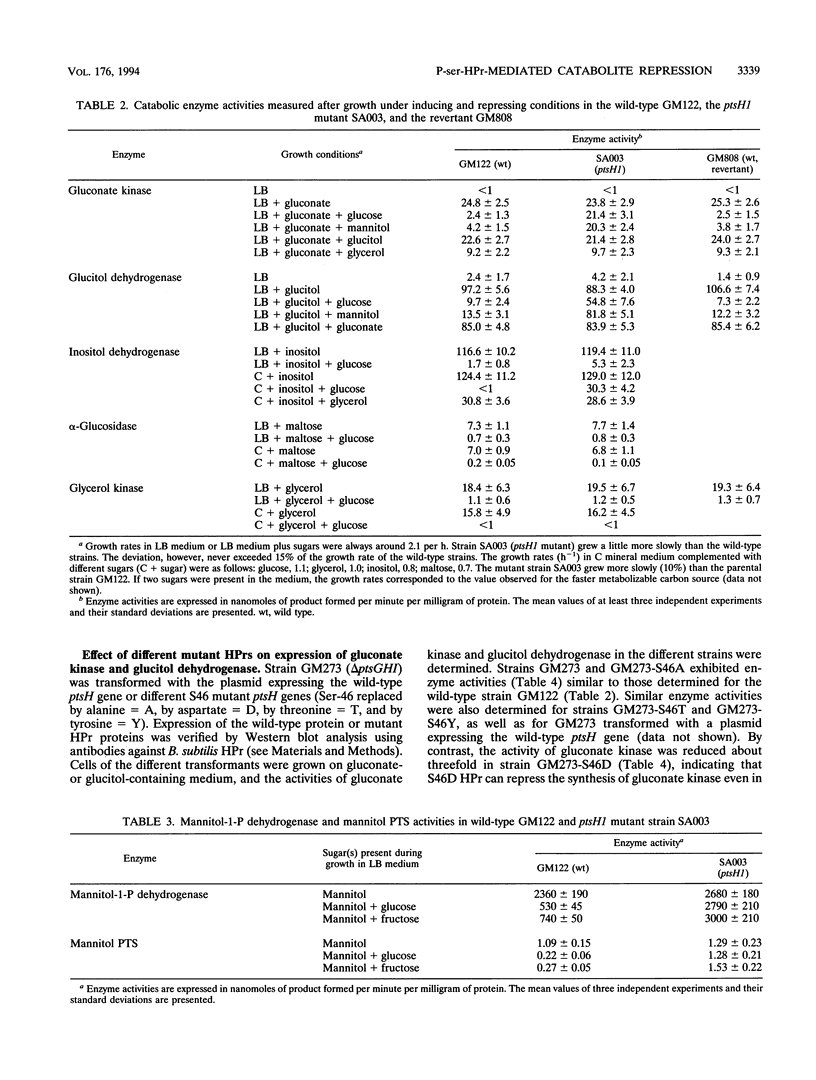
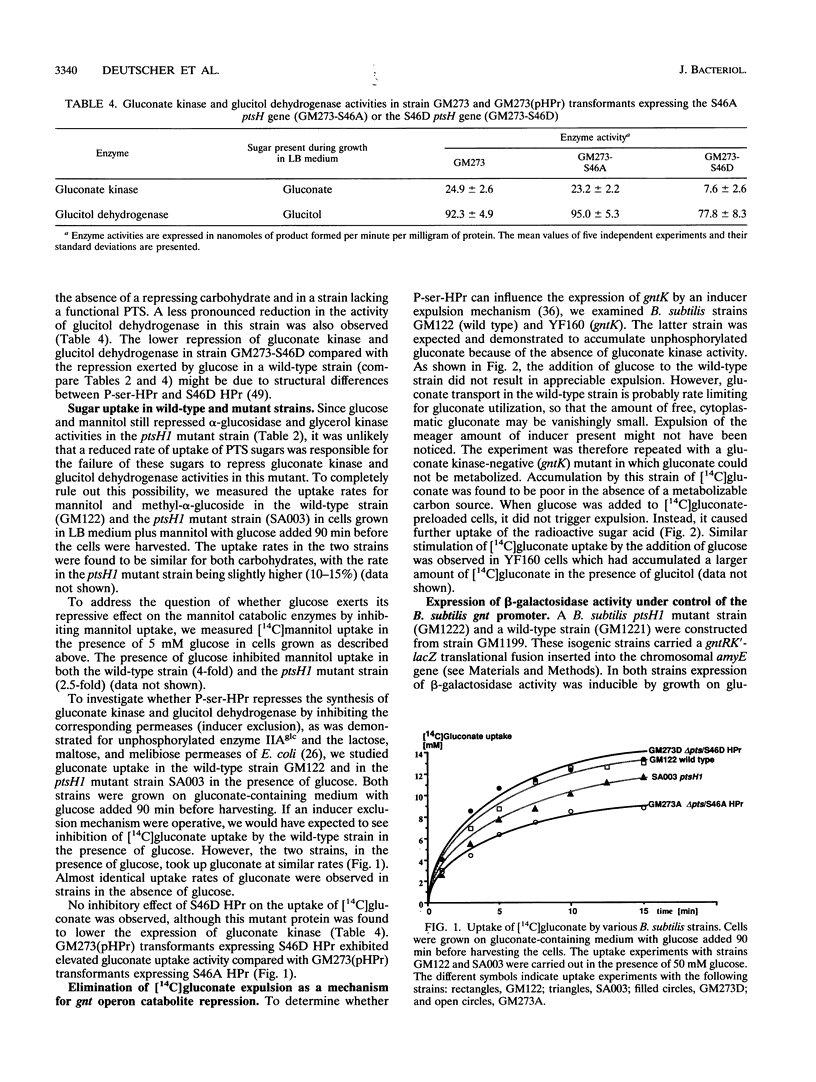
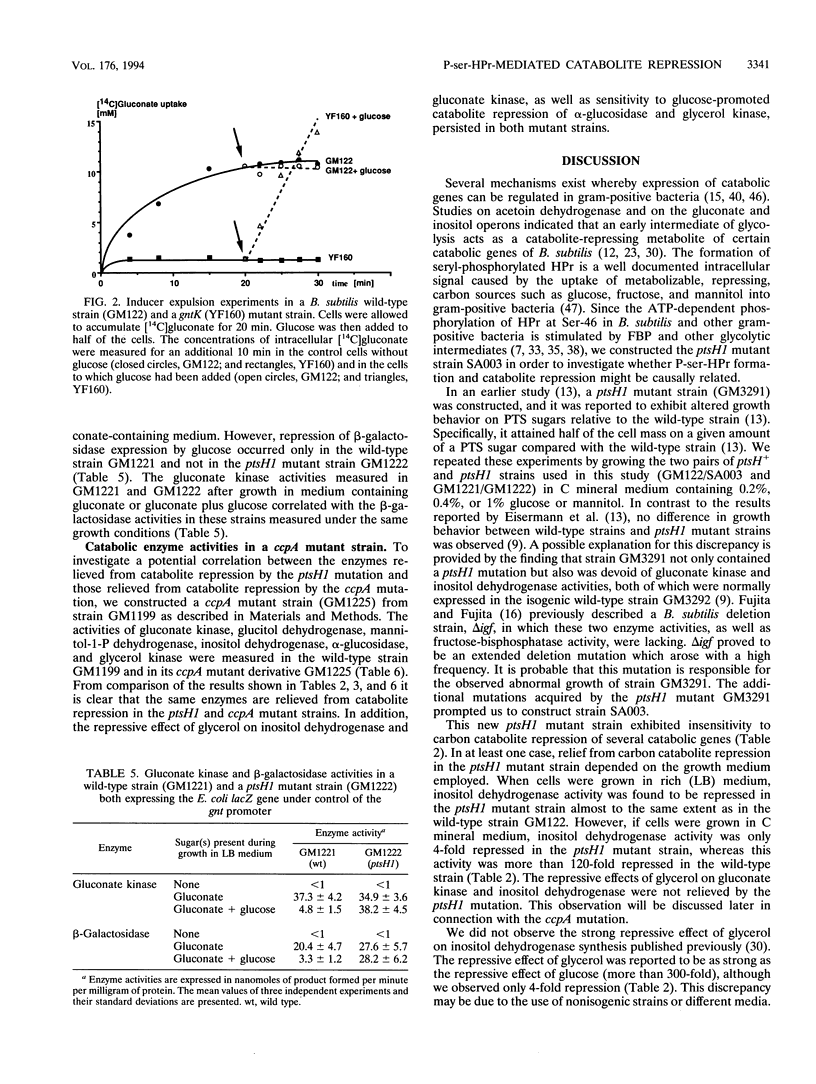
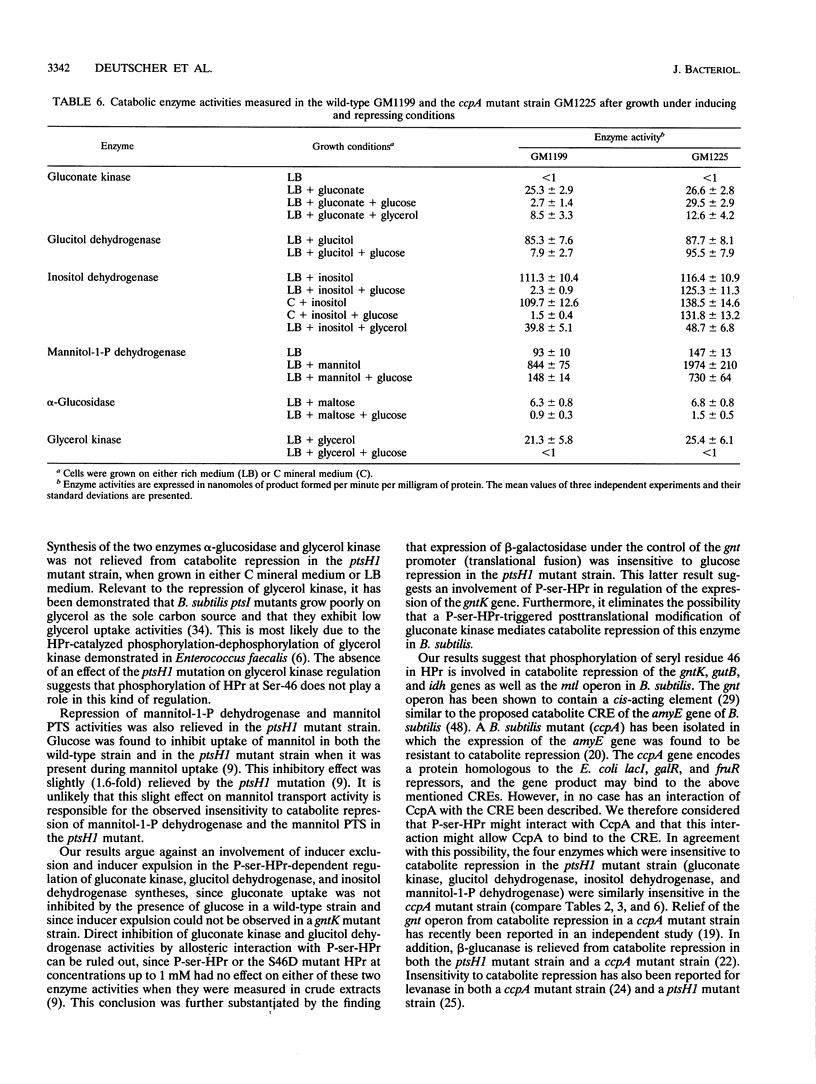
In gram-positive bacteria, HPr, a phosphocarrier protein of the phosphoenolpyruvate:sugar phosphotransferase system (PTS), is phosphorylated by an ATP-dependent, metabolite-activated protein kinase on seryl residue 46. In a Bacillus subtilis mutant strain in which Ser-46 of HPr was replaced with a nonphosphorylatable alanyl residue (ptsH1 mutation), synthesis of gluconate kinase, glucitol dehydrogenase, mannitol-1-P dehydrogenase and the mannitol-specific PTS permease was completely relieved from repression by glucose, fructose, or mannitol, whereas synthesis of inositol dehydrogenase was partially relieved from catabolite repression and synthesis of alpha-glucosidase and glycerol kinase was still subject to catabolite repression. When the S46A mutation in HPr was reverted to give S46 wild-type HPr, expression of gluconate kinase and glucitol dehydrogenase regained full sensitivity to repression by PTS sugars. These results suggest that phosphorylation of HPr at Ser-46 is directly or indirectly involved in catabolite repression. A strain deleted for the ptsGHI genes was transformed with plasmids expressing either the wild-type ptsH gene or various S46 mutant ptsH genes (S46A or S46D). Expression of the gene encoding S46D HPr, having a structure similar to that of P-ser-HPr according to nuclear magnetic resonance data, caused significant reduction of gluconate kinase activity, whereas expression of the genes encoding wild-type or S46A HPr had no effect on this enzyme activity. When the promoterless lacZ gene was put under the control of the gnt promoter and was subsequently incorporated into the amyE gene on the B. subtilis chromosome, expression of beta-galactosidase was inducible by gluconate and repressed by glucose. However, we observed no repression of beta-galactosidase activity in a strain carrying the ptsH1 mutation. Additionally, we investigated a ccpA mutant strain and observed that all of the enzymes which we found to be relieved from carbon catabolite repression in the ptsH1 mutant strain were also insensitive to catabolite repression in the ccpA mutant. Enzymes that were repressed in the ptsH1 mutant were also repressed in the ccpA mutant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniewski C., Savelli B., Stragier P. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J Bacteriol. 1990 Jan;172(1):86–93. doi: 10.1128/jb.172.1.86-93.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymerich S., Gonzy-Tréboul G., Steinmetz M. 5'-noncoding region sacR is the target of all identified regulation affecting the levansucrase gene in Bacillus subtilis. J Bacteriol. 1986 Jun;166(3):993–998. doi: 10.1128/jb.166.3.993-998.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chasin L. A., Magasanik B. Induction and repression of the histidine-degrading enzymes of Bacillus subtilis. J Biol Chem. 1968 Oct 10;243(19):5165–5178. [PubMed] [Google Scholar]
- Crutz A. M., Steinmetz M. Transcription of the Bacillus subtilis sacX and sacY genes, encoding regulators of sucrose metabolism, is both inducible by sucrose and controlled by the DegS-DegU signalling system. J Bacteriol. 1992 Oct;174(19):6087–6095. doi: 10.1128/jb.174.19.6087-6095.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Bauer B., Sauerwald H. Regulation of glycerol metabolism in Enterococcus faecalis by phosphoenolpyruvate-dependent phosphorylation of glycerol kinase catalyzed by enzyme I and HPr of the phosphotransferase system. J Bacteriol. 1993 Jun;175(12):3730–3733. doi: 10.1128/jb.175.12.3730-3733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Pevec B., Beyreuther K., Kiltz H. H., Hengstenberg W. Streptococcal phosphoenolpyruvate-sugar phosphotransferase system: amino acid sequence and site of ATP-dependent phosphorylation of HPr. Biochemistry. 1986 Oct 21;25(21):6543–6551. doi: 10.1021/bi00369a031. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Saier M. H., Jr ATP-dependent protein kinase-catalyzed phosphorylation of a seryl residue in HPr, a phosphate carrier protein of the phosphotransferase system in Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6790–6794. doi: 10.1073/pnas.80.22.6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Sauerwald H. Stimulation of dihydroxyacetone and glycerol kinase activity in Streptococcus faecalis by phosphoenolpyruvate-dependent phosphorylation catalyzed by enzyme I and HPr of the phosphotransferase system. J Bacteriol. 1986 Jun;166(3):829–836. doi: 10.1128/jb.166.3.829-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisermann R., Deutscher J., Gonzy-Treboul G., Hengstenberg W. Site-directed mutagenesis with the ptsH gene of Bacillus subtilis. Isolation and characterization of heat-stable proteins altered at the ATP-dependent regulatory phosphorylation site. J Biol Chem. 1988 Nov 15;263(32):17050–17054. [PubMed] [Google Scholar]
- Ferrari F. A., Ferrari E., Hoch J. A. Chromosomal location of a Bacillus subtilis DNA fragment uniquely transcribed by sigma-28-containing RNA polymerase. J Bacteriol. 1982 Nov;152(2):780–785. doi: 10.1128/jb.152.2.780-785.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Control of carbon and nitrogen metabolism in Bacillus subtilis. Annu Rev Microbiol. 1991;45:107–135. doi: 10.1146/annurev.mi.45.100191.000543. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Fujita T. Effect of mutations causing gluconate kinase or gluconate permease deficiency on expression of the Bacillus subtilis gnt operon. J Bacteriol. 1989 Mar;171(3):1751–1754. doi: 10.1128/jb.171.3.1751-1754.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Fujita T. Genetic analysis of a pleiotropic deletion mutation (delta igf) in Bacillus subtilis. J Bacteriol. 1983 May;154(2):864–869. doi: 10.1128/jb.154.2.864-869.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Fujita T., Miwa Y., Nihashi J., Aratani Y. Organization and transcription of the gluconate operon, gnt, of Bacillus subtilis. J Biol Chem. 1986 Oct 15;261(29):13744–13753. [PubMed] [Google Scholar]
- Fujita Y., Miwa Y. Catabolite repression of the Bacillus subtilis gnt operon mediated by the CcpA protein. J Bacteriol. 1994 Jan;176(2):511–513. doi: 10.1128/jb.176.2.511-513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin T. M., Grundy F. J., Nicholson W. L., Chambliss G. H. Catabolite repression of alpha-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacl and galR repressors. Mol Microbiol. 1991 Mar;5(3):575–584. doi: 10.1111/j.1365-2958.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Jalanko A., Palva I., Söderlund Restriction maps of plasmids pUB110 and pBD9. Gene. 1981 Sep;14(4):325–328. doi: 10.1016/0378-1119(81)90165-7. [DOI] [PubMed] [Google Scholar]
- Krüger S., Stülke J., Hecker M. Catabolite repression of beta-glucanase synthesis in Bacillus subtilis. J Gen Microbiol. 1993 Sep;139(9):2047–2054. doi: 10.1099/00221287-139-9-2047. [DOI] [PubMed] [Google Scholar]
- Lopez J. M., Thoms B. Role of sugar uptake and metabolic intermediates on catabolite repression in Bacillus subtilis. J Bacteriol. 1977 Jan;129(1):217–224. doi: 10.1128/jb.129.1.217-224.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I., Debarbouille M., Klier A., Rapoport G. Induction and metabolite regulation of levanase synthesis in Bacillus subtilis. J Bacteriol. 1989 Apr;171(4):1885–1892. doi: 10.1128/jb.171.4.1885-1892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow N. D., Fox D. K., Roseman S. The bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Biochem. 1990;59:497–542. doi: 10.1146/annurev.bi.59.070190.002433. [DOI] [PubMed] [Google Scholar]
- Miwa Y., Fujita Y. Determination of the cis sequence involved in catabolite repression of the Bacillus subtilis gnt operon; implication of a consensus sequence in catabolite repression in the genus Bacillus. Nucleic Acids Res. 1990 Dec 11;18(23):7049–7053. doi: 10.1093/nar/18.23.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa Y., Fujita Y. Efficient utilization and operation of the gluconate-inducible system of the promoter of the Bacillus subtilis gnt operon in Escherichia coli. J Bacteriol. 1987 Nov;169(11):5333–5335. doi: 10.1128/jb.169.11.5333-5335.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihashi J., Fujita Y. Catabolite repression of inositol dehydrogenase and gluconate kinase syntheses in Bacillus subtilis. Biochim Biophys Acta. 1984 Mar 22;798(1):88–95. doi: 10.1016/0304-4165(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Novotny M. J., Reizer J., Esch F., Saier M. H., Jr Purification and properties of D-mannitol-1-phosphate dehydrogenase and D-glucitol-6-phosphate dehydrogenase from Escherichia coli. J Bacteriol. 1984 Sep;159(3):986–990. doi: 10.1128/jb.159.3.986-990.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiche B., Frank R., Deutscher J., Meyer N., Hengstenberg W. Staphylococcal phosphoenolpyruvate-dependent phosphotransferase system: purification and characterization of the mannitol-specific enzyme IIImtl of Staphylococcus aureus and Staphylococcus carnosus and homology with the enzyme IImtl of Escherichia coli. Biochemistry. 1988 Aug 23;27(17):6512–6516. doi: 10.1021/bi00417a047. [DOI] [PubMed] [Google Scholar]
- Reizer J., Novotny M. J., Hengstenberg W., Saier M. H., Jr Properties of ATP-dependent protein kinase from Streptococcus pyogenes that phosphorylates a seryl residue in HPr, a phosphocarrier protein of the phosphotransferase system. J Bacteriol. 1984 Oct;160(1):333–340. doi: 10.1128/jb.160.1.333-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Panos C. Regulation of beta-galactoside phosphate accumulation in Streptococcus pyogenes by an expulsion mechanism. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5497–5501. doi: 10.1073/pnas.77.9.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Peterkofsky A., Romano A. H. Evidence for the presence of heat-stable protein (HPr) and ATP-dependent HPr kinase in heterofermentative lactobacilli lacking phosphoenolpyruvate:glycose phosphotransferase activity. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2041–2045. doi: 10.1073/pnas.85.7.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr, Deutscher J., Grenier F., Thompson J., Hengstenberg W. The phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: properties, mechanism, and regulation. Crit Rev Microbiol. 1988;15(4):297–338. doi: 10.3109/10408418809104461. [DOI] [PubMed] [Google Scholar]
- Reizer J., Sutrina S. L., Saier M. H., Stewart G. C., Peterkofsky A., Reddy P. Mechanistic and physiological consequences of HPr(ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J. 1989 Jul;8(7):2111–2120. doi: 10.1002/j.1460-2075.1989.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr A multiplicity of potential carbon catabolite repression mechanisms in prokaryotic and eukaryotic microorganisms. New Biol. 1991 Dec;3(12):1137–1147. [PubMed] [Google Scholar]
- Shimotsu H., Henner D. J. Construction of a single-copy integration vector and its use in analysis of regulation of the trp operon of Bacillus subtilis. Gene. 1986;43(1-2):85–94. doi: 10.1016/0378-1119(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Le Coq D., Aymerich S. Induction of saccharolytic enzymes by sucrose in Bacillus subtilis: evidence for two partially interchangeable regulatory pathways. J Bacteriol. 1989 Mar;171(3):1519–1523. doi: 10.1128/jb.171.3.1519-1523.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. C. Catabolite repression in the gram-positive bacteria: generation of negative regulators of transcription. J Cell Biochem. 1993 Jan;51(1):25–28. doi: 10.1002/jcb.240510106. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Brochu D., Reizer J. Quantitative determination of the intracellular concentration of the various forms of HPr, a phosphocarrier protein of the phosphoenolpyruvate: sugar phosphotransferase system in growing cells of oral streptococci. Anal Biochem. 1991 Jul;196(1):24–30. doi: 10.1016/0003-2697(91)90112-7. [DOI] [PubMed] [Google Scholar]
- Weickert M. J., Chambliss G. H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekind M., Reizer J., Deutscher J., Saier M. H., Klevit R. E. Common structural changes accompany the functional inactivation of HPr by seryl phosphorylation or by serine to aspartate substitution. Biochemistry. 1989 Dec 26;28(26):9908–9912. doi: 10.1021/bi00452a005. [DOI] [PubMed] [Google Scholar]


