Abstract
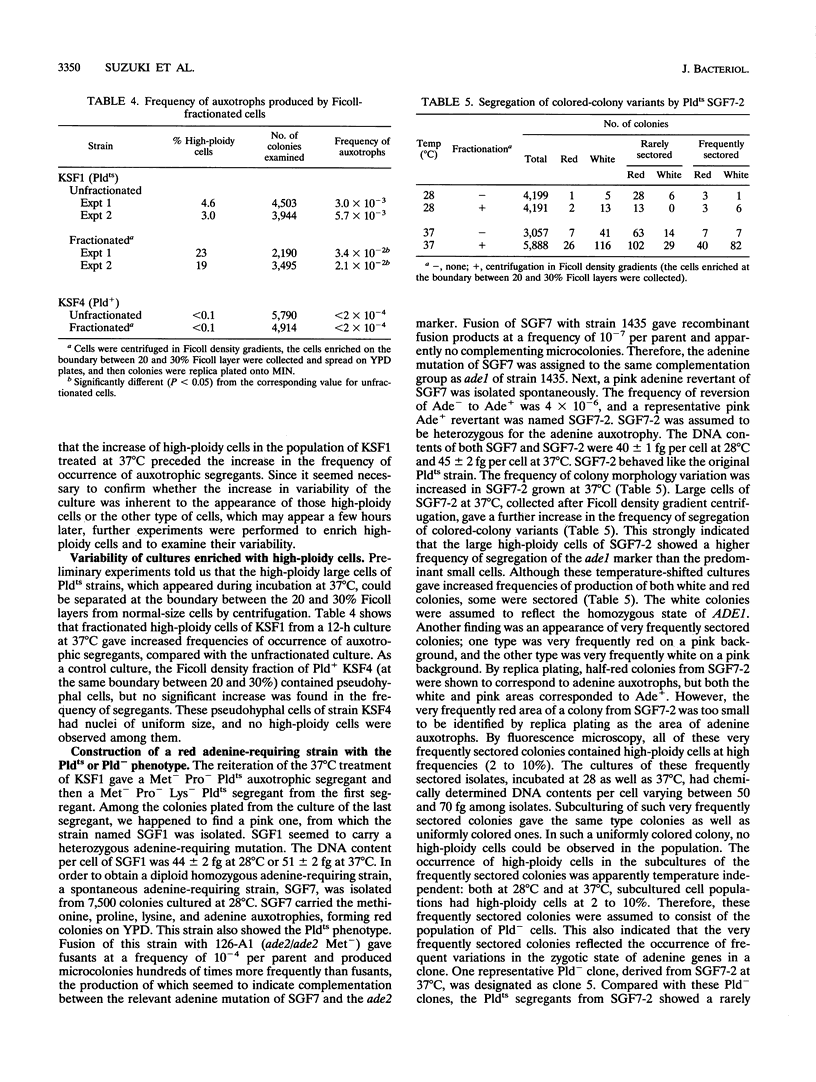
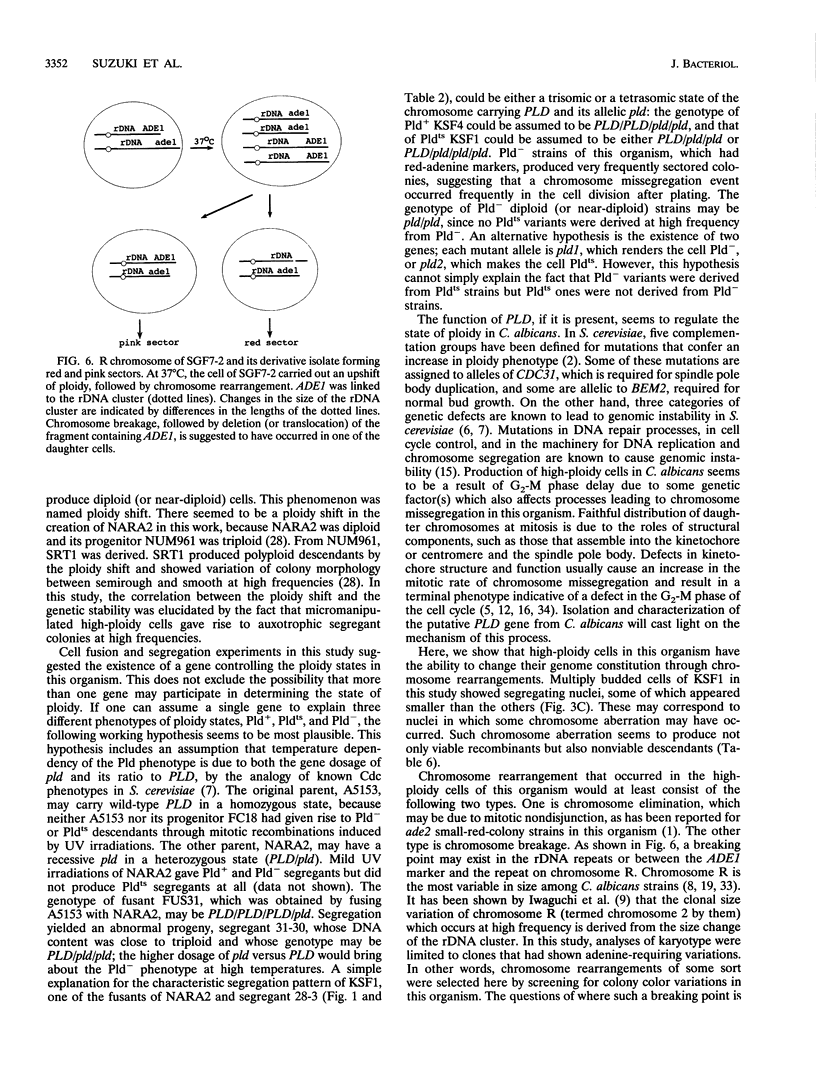
In order to clarify the relationship between polyploidization and the capability of phenotypic switching in the imperfect yeast Candida albicans, two types of variants were isolated as segregants from a fusant, which produced a proportion of the cell population with a higher ploidy than the rest, either in a temperature-dependent or -independent manner, when incubated at low (28 degrees C) and high (37 degrees C) temperatures. In the case of the temperature-dependent type of variants, high-ploidy cells appeared at 37 degrees C but rarely at 28 degrees C. This phenotype was named Pldts (temperature-sensitive polyploidization), and the temperature-independent phenotype was called Pld-. The appearance of high-ploidy cells in the culture of the Pldts strain at 37 degrees C was accompanied by a significant increase in the frequency of auxotrophic variants; these variants probably occur as a result of segregation of auxotrophic markers from the heterozygous to the homozygous state. Both Pldts and Pld- phenotypes were recessive in a fusion with a Pld+ parent. An adenine auxotrophic marker (ade1) was introduced into a Pldts strain in a heterozygous state, and the individual high-ploidy cells of this strain, grown at 37 degrees C, were micromanipulated to form colonies, which consisted of red and white sectors appearing at high frequency on a pink background. When the ade1 auxotrophy was introduced into Pld- strains, frequently sectored colonies were produced. These results suggested an increased level of chromosome missegregation in both types of Pld mutants. Analyses by pulsed-field gel electrophoresis of Ade-segregants, derived from a micromanipulated high-ploidy cell of a Pld(ts) strain, suggested the occurrence of nonreciprocal recombination, some of which includes chromosome loss.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton R. C., Gull K. Isolation, characterization, and genetic analysis of monosomic, aneuploid mutants of Candida albicans. Mol Microbiol. 1992 Jan;6(2):171–177. doi: 10.1111/j.1365-2958.1992.tb01998.x. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993 Nov;135(3):677–691. doi: 10.1093/genetics/135.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W. S., Magee B. B., Magee P. T. Construction of an SfiI macrorestriction map of the Candida albicans genome. J Bacteriol. 1993 Oct;175(20):6637–6651. doi: 10.1128/jb.175.20.6637-6651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W. S., Rikkerink E. H., Magee P. T. Genetics of the white-opaque transition in Candida albicans: demonstration of switching recessivity and mapping of switching genes. J Bacteriol. 1992 May;174(9):2951–2957. doi: 10.1128/jb.174.9.2951-2957.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doheny K. F., Sorger P. K., Hyman A. A., Tugendreich S., Spencer F., Hieter P. Identification of essential components of the S. cerevisiae kinetochore. Cell. 1993 May 21;73(4):761–774. doi: 10.1016/0092-8674(93)90255-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985 Jul;110(3):381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992 Nov 13;71(4):543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- Iwaguchi S., Homma M., Tanaka K. Clonal variation of chromosome size derived from the rDNA cluster region in Candida albicans. J Gen Microbiol. 1992 Jun;138(6):1177–1184. doi: 10.1099/00221287-138-6-1177. [DOI] [PubMed] [Google Scholar]
- Iwaguchi S., Homma M., Tanaka K. Variation in the electrophoretic karyotype analysed by the assignment of DNA probes in Candida albicans. J Gen Microbiol. 1990 Dec;136(12):2433–2442. doi: 10.1099/00221287-136-12-2433. [DOI] [PubMed] [Google Scholar]
- Kakar S. N., Partridge R. M., Magee P. T. A genetic analysis of Candida albicans: isolation of a wide variety of auxotrophs and demonstration of linkage and complementation. Genetics. 1983 Jun;104(2):241–255. doi: 10.1093/genetics/104.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouprina N., Kirillov A., Kroll E., Koryabin M., Shestopalov B., Bannikov V., Zakharyev V., Larionov V. Identification and cloning of the CHL4 gene controlling chromosome segregation in yeast. Genetics. 1993 Oct;135(2):327–341. doi: 10.1093/genetics/135.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Kirsch D. R. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol Cell Biol. 1986 Jan;6(1):142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee B. B., Magee P. T. Electrophoretic karyotypes and chromosome numbers in Candida species. J Gen Microbiol. 1987 Feb;133(2):425–430. doi: 10.1099/00221287-133-2-425. [DOI] [PubMed] [Google Scholar]
- Palmer R. E., Hogan E., Koshland D. Mitotic transmission of artificial chromosomes in cdc mutants of the yeast, Saccharomyces cerevisiae. Genetics. 1990 Aug;125(4):763–774. doi: 10.1093/genetics/125.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périer F., Carbon J. A colony color assay for Saccharomyces cerevisiae mutants defective in kinetochore structure and function. Genetics. 1992 Sep;132(1):39–51. doi: 10.1093/genetics/132.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggsby W. S., Torres-Bauza L. J., Wills J. W., Townes T. M. DNA content, kinetic complexity, and the ploidy question in Candida albicans. Mol Cell Biol. 1982 Jul;2(7):853–862. doi: 10.1128/mcb.2.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikkerink E. H., Magee B. B., Magee P. T. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988 Feb;170(2):895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustchenko-Bulgac E. P., Sherman F., Hicks J. B. Chromosomal rearrangements associated with morphological mutants provide a means for genetic variation of Candida albicans. J Bacteriol. 1990 Mar;172(3):1276–1283. doi: 10.1128/jb.172.3.1276-1283.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustchenko-Bulgac E. P. Variations of Candida albicans electrophoretic karyotypes. J Bacteriol. 1991 Oct;173(20):6586–6596. doi: 10.1128/jb.173.20.6586-6596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustchenko E. P., Curran T. M., Sherman F. Variations in the number of ribosomal DNA units in morphological mutants and normal strains of Candida albicans and in normal strains of Saccharomyces cerevisiae. J Bacteriol. 1993 Nov;175(22):7189–7199. doi: 10.1128/jb.175.22.7189-7199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Magee P. T. Genetics of Candida albicans. Microbiol Rev. 1990 Sep;54(3):226–241. doi: 10.1128/mr.54.3.226-241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Kanbe T., Kuroiwa T., Tanaka K. Occurrence of ploidy shift in a strain of the imperfect yeast Candida albicans. J Gen Microbiol. 1986 Feb;132(2):443–453. doi: 10.1099/00221287-132-2-443. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kobayashi I., Kanbe T., Tanaka K. High frequency variation of colony morphology and chromosome reorganization in the pathogenic yeast Candida albicans. J Gen Microbiol. 1989 Feb;135(Pt 2):425–434. doi: 10.1099/00221287-135-2-425. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Miyamae Y., Ishida I. Variation of colony morphology and chromosomal rearrangement in Candida tropicalis pK233. J Gen Microbiol. 1991 Jan;137(1):161–167. doi: 10.1099/00221287-137-1-161. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Rogers A. L., Magee P. T. Inter- and intra-species crosses between Candida albicans and Candida guilliermondii. Yeast. 1986 Mar;2(1):53–58. doi: 10.1002/yea.320020104. [DOI] [PubMed] [Google Scholar]
- Whelan W. L., Partridge R. M., Magee P. T. Heterozygosity and segregation in Candida albicans. Mol Gen Genet. 1980;180(1):107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]
- Wickes B., Staudinger J., Magee B. B., Kwon-Chung K. J., Magee P. T., Scherer S. Physical and genetic mapping of Candida albicans: several genes previously assigned to chromosome 1 map to chromosome R, the rDNA-containing linkage group. Infect Immun. 1991 Jul;59(7):2480–2484. doi: 10.1128/iai.59.7.2480-2484.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z., McGrew J. T., Schroeder A. J., Fitzgerald-Hayes M. CSE1 and CSE2, two new genes required for accurate mitotic chromosome segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Aug;13(8):4691–4702. doi: 10.1128/mcb.13.8.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt J. P., Pitout M. J. Ploidy differences in Sporobolomyces salmonicolor and Candida albicans. Antonie Van Leeuwenhoek. 1969;35(2):227–231. [PubMed] [Google Scholar]