Abstract
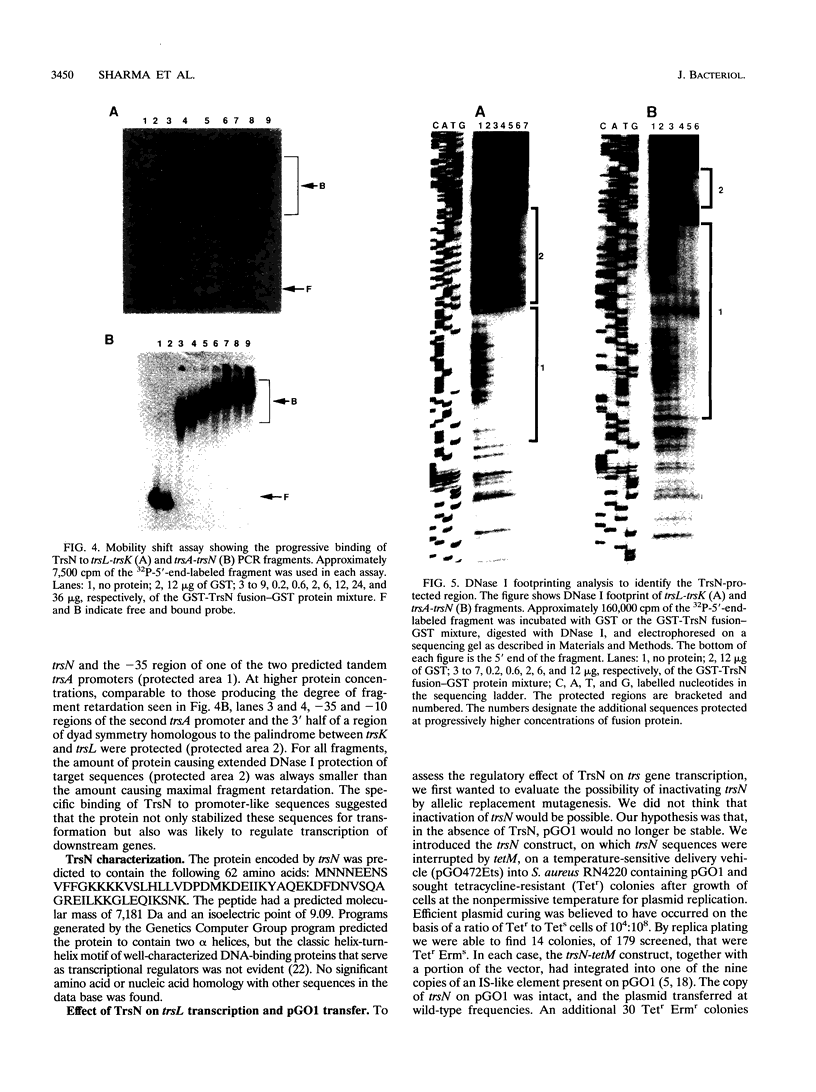
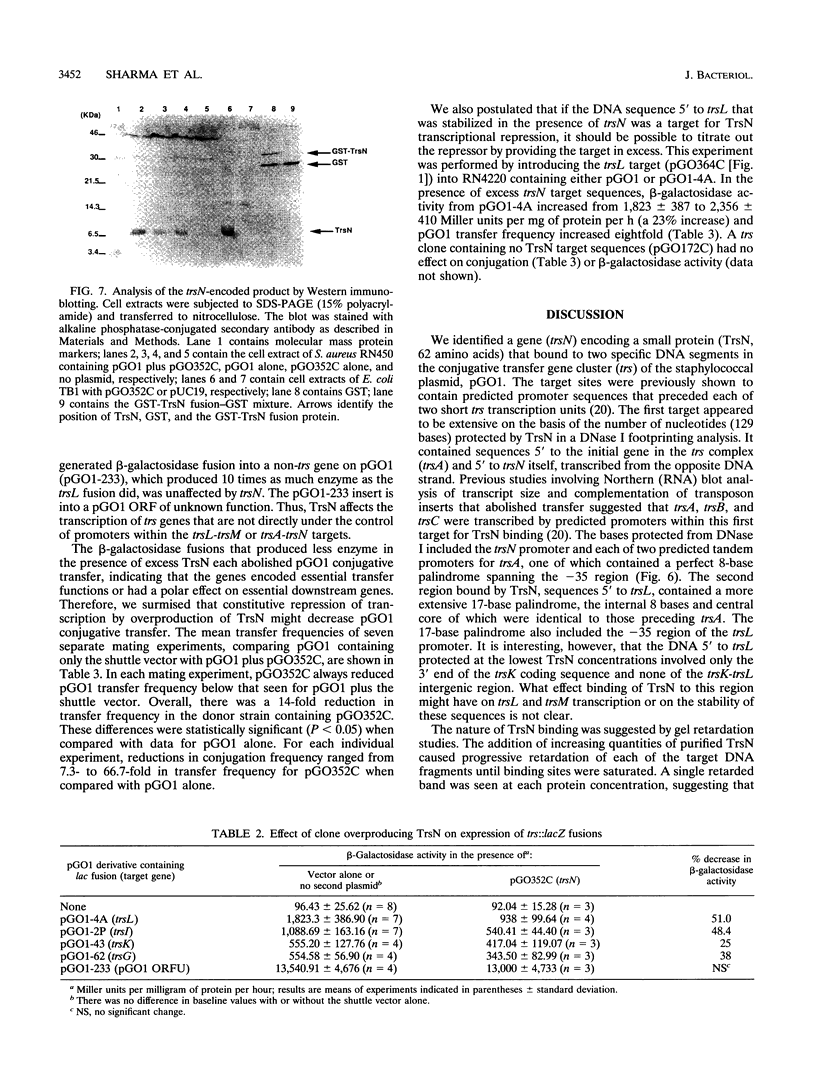
The major conjugative transfer gene cluster of staphylococcal plasmid pGO1 (trs) consists of 13 open reading frames (trsA to trsM) transcribed from one DNA strand and a single 189-bp open reading frame (trsN) within the first 348 bp of trs that is transcribed divergently. Promoter regions for trsN and trsA partially overlap. TrsN, a 7,181-Da protein, was purified as a fusion to glutathione S-transferase and found to have DNA-binding activity. Increasing concentrations of the fusion protein progressively retarded the gel migration of PCR-generated DNA fragments containing predicted promoters 5' to trsL, trsA, and trsN. The target sequences contained areas of identity, including regions of dyad symmetry, that were protected in DNase I footprinting studies. The binding of TrsN to its trsL target was required for this target DNA to be stably introduced into Staphylococcus aureus on a high-copy-number vector. Provision of excess TrsN from this high-copy-number vector in S. aureus decreased beta-galactosidase activity from a trsL-lacZ transcriptional fusion and decreased pGO1 conjugation frequency. Conversely, both transcription and conjugation increased in the presence of excess trsL target. We propose that TrsN negatively regulates the transcription of genes essential for conjugative transfer by binding to regions 5' to their translational start sites.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Byrne M. E., Gillespie M. T., Skurray R. A. Molecular analysis of a gentamicin resistance transposonlike element on plasmids isolated from North American Staphylococcus aureus strains. Antimicrob Agents Chemother. 1990 Nov;34(11):2106–2113. doi: 10.1128/aac.34.11.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Bacterial sex pheromone-induced plasmid transfer. Cell. 1993 Apr 9;73(1):9–12. doi: 10.1016/0092-8674(93)90153-h. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K. A., Minkley E. G., Jr The conjugation system of F, the fertility factor of Escherichia coli. Annu Rev Genet. 1986;20:593–624. doi: 10.1146/annurev.ge.20.120186.003113. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Lane D., Prentki P., Chandler M. Use of gel retardation to analyze protein-nucleic acid interactions. Microbiol Rev. 1992 Dec;56(4):509–528. doi: 10.1128/mr.56.4.509-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessl M., Balzer D., Lurz R., Waters V. L., Guiney D. G., Lanka E. Dissection of IncP conjugative plasmid transfer: definition of the transfer region Tra2 by mobilization of the Tra1 region in trans. J Bacteriol. 1992 Apr;174(8):2493–2500. doi: 10.1128/jb.174.8.2493-2500.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessl M., Balzer D., Pansegrau W., Lanka E. Sequence similarities between the RP4 Tra2 and the Ti VirB region strongly support the conjugation model for T-DNA transfer. J Biol Chem. 1992 Oct 5;267(28):20471–20480. [PubMed] [Google Scholar]
- Morton T. M., Eaton D. M., Johnston J. L., Archer G. L. DNA sequence and units of transcription of the conjugative transfer gene complex (trs) of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1993 Jul;175(14):4436–4447. doi: 10.1128/jb.175.14.4436-4447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Pontius L. T., Clewell D. B. Conjugative transfer of Enterococcus faecalis plasmid pAD1: nucleotide sequence and transcriptional fusion analysis of a region involved in positive regulation. J Bacteriol. 1992 May;174(10):3152–3160. doi: 10.1128/jb.174.10.3152-3160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Identification and cloning of the conjugative transfer region of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1989 Feb;171(2):684–691. doi: 10.1128/jb.171.2.684-691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K. E., Clewell D. B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J Bacteriol. 1988 Sep;170(9):4343–4352. doi: 10.1128/jb.170.9.4343-4352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young C., Prince A. S., Figurski D. H. korA function of promiscuous plasmid RK2: an autorepressor that inhibits expression of host-lethal gene kilA and replication gene trfA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7374–7378. doi: 10.1073/pnas.82.21.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bergh E. R., Dijkhuizen L., Meijer W. G. CbbR, a LysR-type transcriptional activator, is required for expression of the autotrophic CO2 fixation enzymes of Xanthobacter flavus. J Bacteriol. 1993 Oct;175(19):6097–6104. doi: 10.1128/jb.175.19.6097-6104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]