Abstract
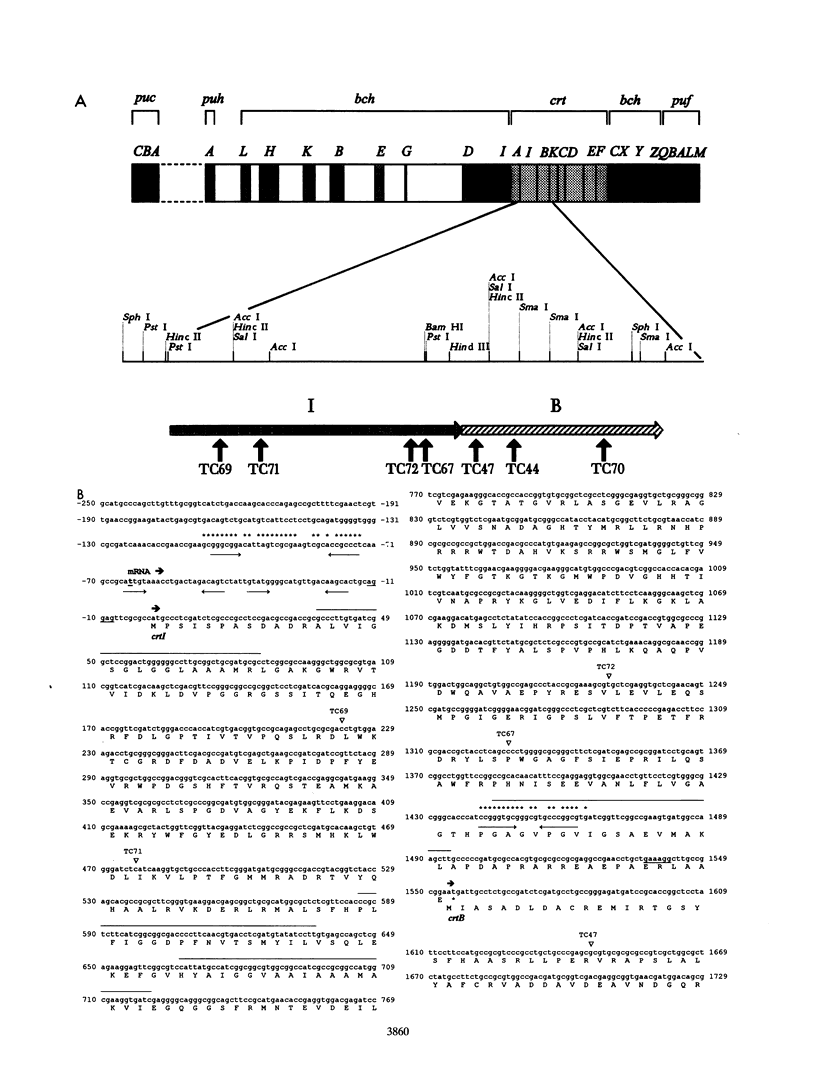
In the purple photosynthetic bacterium Rhodobacter sphaeroides, the desaturation of phytoene has already been implicated in the assembly of the light-harvesting 2 complex (H.P. Lang and C.N. Hunter, Biochem. J. 298:197-205, 1994). The phytoene synthase and desaturase enzymes mediate the first steps specific for carotenoid biosynthesis up to and including the synthesis of the colored carotenoid neurosporene. In this report, we present the DNA and deduced amino acid sequences of the genes encoding these proteins, namely, crtB and crtI, from R. sphaeroides and present evidence for the existence of a crtIB operon. Both genes have been shown to possess putative puc and puf operon-like promoter sequences, and oxygen regulation and the point of initiation of the crtI transcript have been demonstrated. The complete crtI gene has been overexpressed in Escherichia coli and R. sphaeroides and shown to catalyze three desaturations of phytoene to give neurosporene. This activity was shown to be ATP dependent, and the cofactor requirement was investigated by using a spectroscopic assay for in vitro carotenogenic activity. Although the crtI and crtB genes have been sequenced from a number of different organisms, the transcriptional organization and regulation of these genes have not been analyzed in detail. In this report, we have located the transcription initiation point and have shown that R. sphaeroides possesses an oxygen-regulated CrtI-type phytoene desaturase gene that forms a transcriptional operon with crtB.
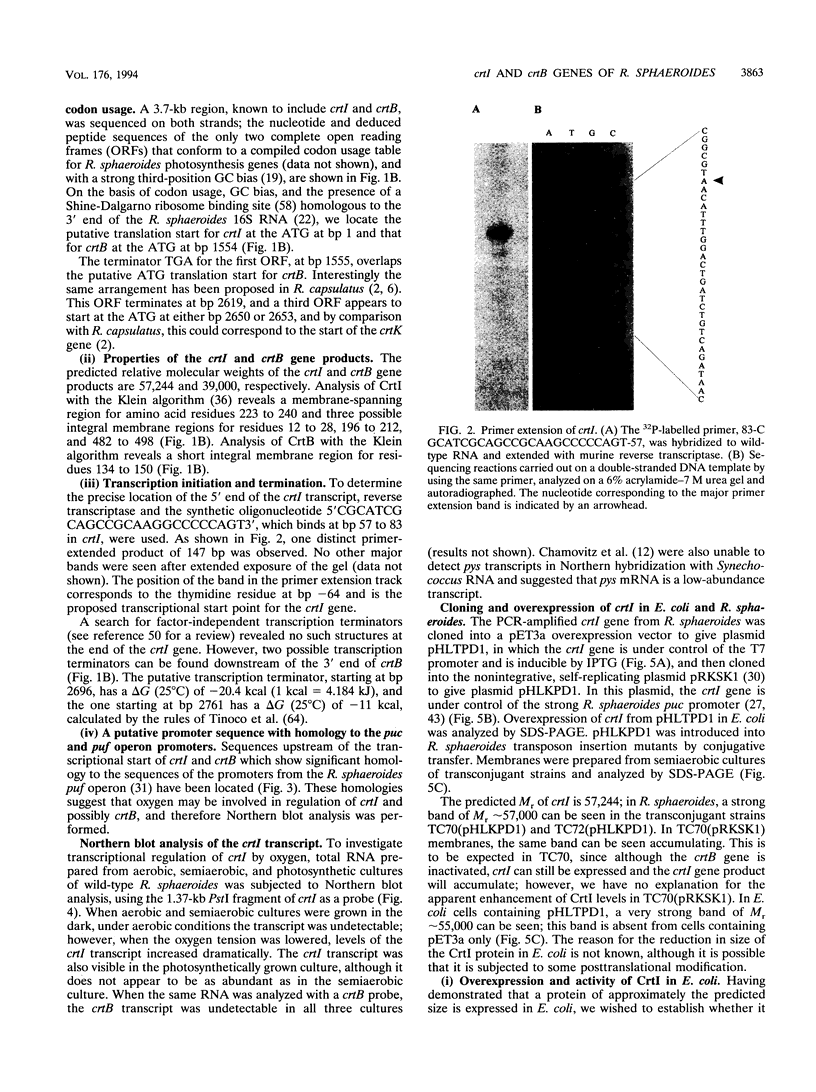
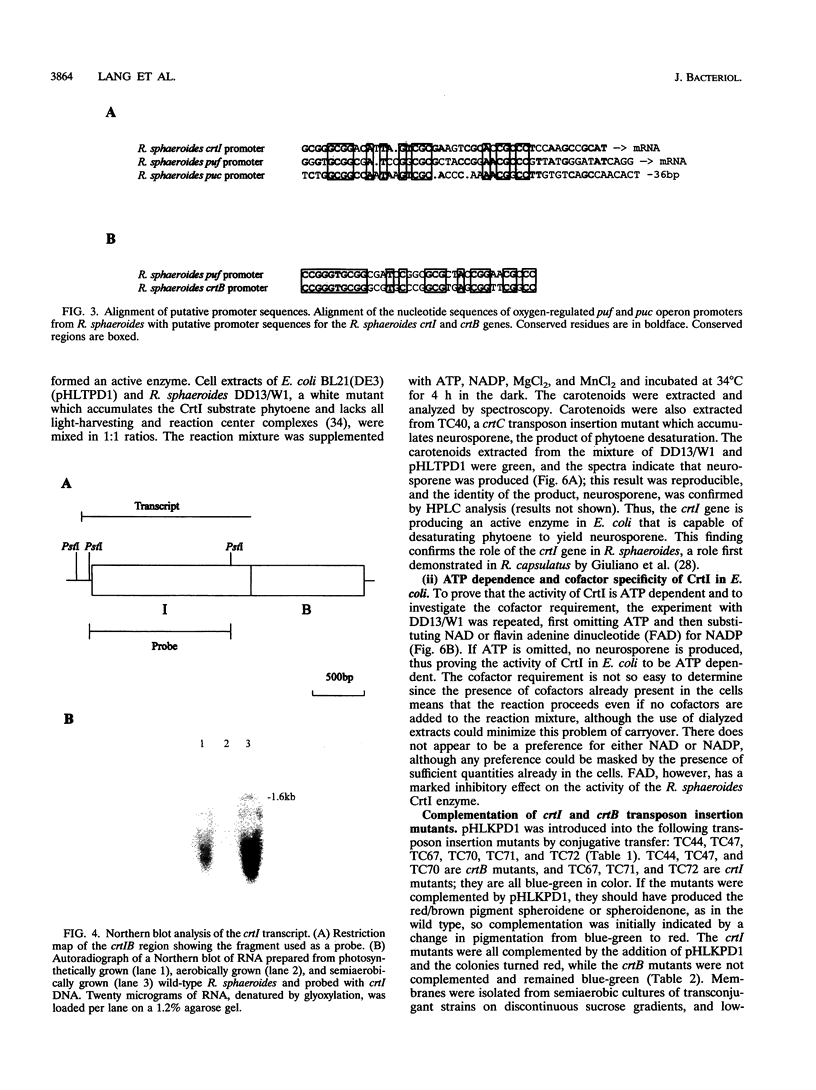
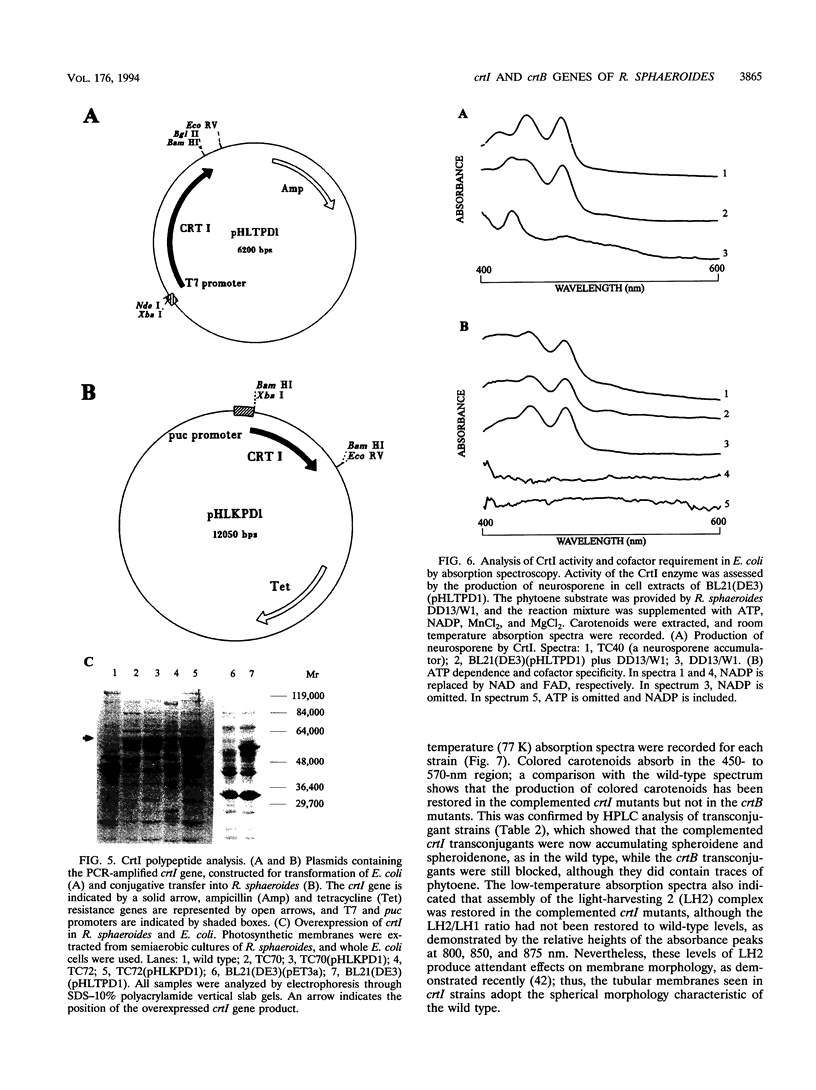
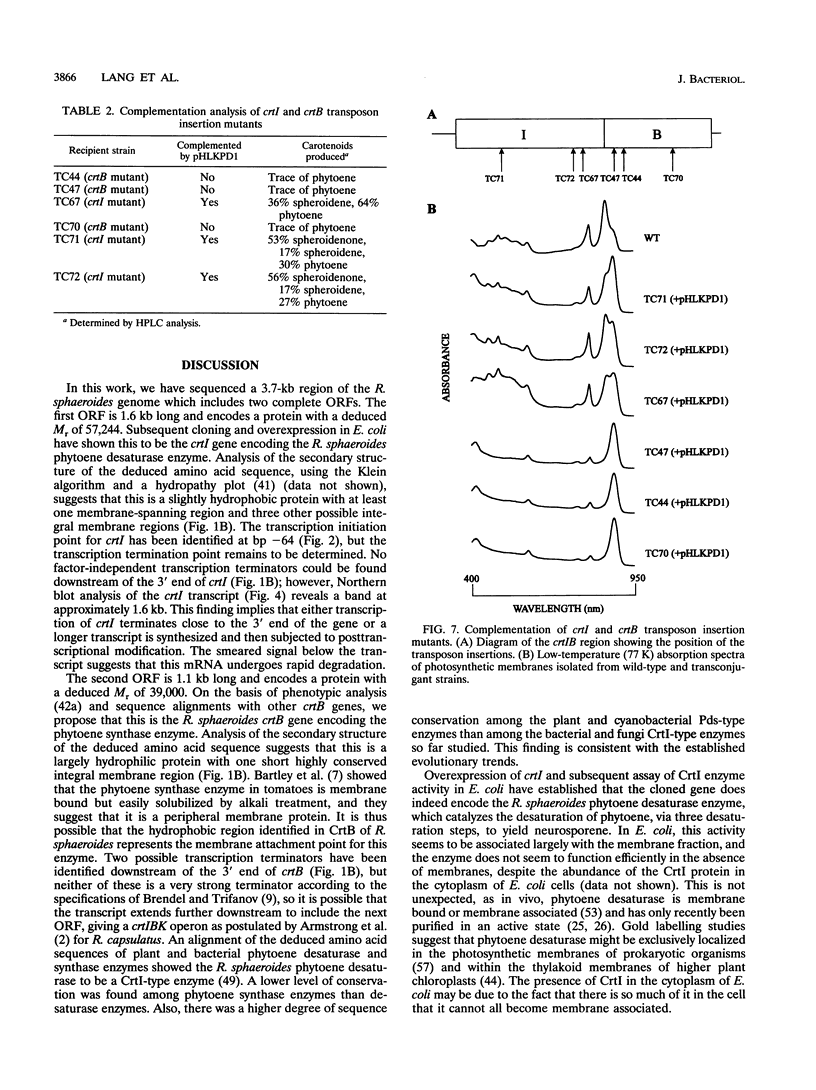
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. doi: 10.1073/pnas.84.17.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong G. A., Alberti M., Leach F., Hearst J. E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet. 1989 Apr;216(2-3):254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- Armstrong G. A., Hundle B. S., Hearst J. E. Evolutionary conservation and structural similarities of carotenoid biosynthesis gene products from photosynthetic and nonphotosynthetic organisms. Methods Enzymol. 1993;214:297–311. doi: 10.1016/0076-6879(93)14073-r. [DOI] [PubMed] [Google Scholar]
- Armstrong G. A., Schmidt A., Sandmann G., Hearst J. E. Genetic and biochemical characterization of carotenoid biosynthesis mutants of Rhodobacter capsulatus. J Biol Chem. 1990 May 15;265(14):8329–8338. [PubMed] [Google Scholar]
- Bartley G. E., Scolnik P. A. Carotenoid biosynthesis in photosynthetic bacteria. Genetic characterization of the Rhodobacter capsulatus CrtI protein. J Biol Chem. 1989 Aug 5;264(22):13109–13113. [PubMed] [Google Scholar]
- Bartley G. E., Viitanen P. V., Bacot K. O., Scolnik P. A. A tomato gene expressed during fruit ripening encodes an enzyme of the carotenoid biosynthesis pathway. J Biol Chem. 1992 Mar 15;267(8):5036–5039. [PubMed] [Google Scholar]
- Bartley G. E., Viitanen P. V., Pecker I., Chamovitz D., Hirschberg J., Scolnik P. A. Molecular cloning and expression in photosynthetic bacteria of a soybean cDNA coding for phytoene desaturase, an enzyme of the carotenoid biosynthesis pathway. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6532–6536. doi: 10.1073/pnas.88.15.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel V., Trifonov E. N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984 May 25;12(10):4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Chamovitz D., Misawa N., Sandmann G., Hirschberg J. Molecular cloning and expression in Escherichia coli of a cyanobacterial gene coding for phytoene synthase, a carotenoid biosynthesis enzyme. FEBS Lett. 1992 Jan 27;296(3):305–310. doi: 10.1016/0014-5793(92)80310-d. [DOI] [PubMed] [Google Scholar]
- Chamovitz D., Pecker I., Hirschberg J. The molecular basis of resistance to the herbicide norflurazon. Plant Mol Biol. 1991 Jun;16(6):967–974. doi: 10.1007/BF00016069. [DOI] [PubMed] [Google Scholar]
- Chang C. H., el-Kabbani O., Tiede D., Norris J., Schiffer M. Structure of the membrane-bound protein photosynthetic reaction center from Rhodobacter sphaeroides. Biochemistry. 1991 Jun 4;30(22):5352–5360. doi: 10.1021/bi00236a005. [DOI] [PubMed] [Google Scholar]
- Coomber S. A., Chaudhri M., Connor A., Britton G., Hunter C. N. Localized transposon Tn5 mutagenesis of the photosynthetic gene cluster of Rhodobacter sphaeroides. Mol Microbiol. 1990 Jun;4(6):977–989. doi: 10.1111/j.1365-2958.1990.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogbo O., Laferriére A., D'Harlingue A., Camara B. Carotenoid biosynthesis: Isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7054–7058. doi: 10.1073/pnas.85.19.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden S. C., Kaplan S. Localization and structural analysis of the ribosomal RNA operons of Rhodobacter sphaeroides. Nucleic Acids Res. 1990 Dec 25;18(24):7267–7277. doi: 10.1093/nar/18.24.7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fraley R. T., Lueking D. R., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Polypeptide insertion into growing membrane. J Biol Chem. 1978 Jan 25;253(2):458–464. [PubMed] [Google Scholar]
- Fraser P. D., Linden H., Sandmann G. Purification and reactivation of recombinant Synechococcus phytoene desaturase from an overexpressing strain of Escherichia coli. Biochem J. 1993 May 1;291(Pt 3):687–692. doi: 10.1042/bj2910687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P. D., Misawa N., Linden H., Yamano S., Kobayashi K., Sandmann G. Expression in Escherichia coli, purification, and reactivation of the recombinant Erwinia uredovora phytoene desaturase. J Biol Chem. 1992 Oct 5;267(28):19891–19895. [PubMed] [Google Scholar]
- Gibson L. C., McGlynn P., Chaudhri M., Hunter C. N. A putative anaerobic coproporphyrinogen III oxidase in Rhodobacter sphaeroides. II. Analysis of a region of the genome encoding hemF and the puc operon. Mol Microbiol. 1992 Nov;6(21):3171–3186. doi: 10.1111/j.1365-2958.1992.tb01773.x. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Pollock D., Scolnik P. A. The gene crtI mediates the conversion of phytoene into colored carotenoids in Rhodopseudomonas capsulata. J Biol Chem. 1986 Oct 5;261(28):12925–12929. [PubMed] [Google Scholar]
- Hunter C. N., Hundle B. S., Hearst J. E., Lang H. P., Gardiner A. T., Takaichi S., Cogdell R. J. Introduction of new carotenoids into the bacterial photosynthetic apparatus by combining the carotenoid biosynthetic pathways of Erwinia herbicola and Rhodobacter sphaeroides. J Bacteriol. 1994 Jun;176(12):3692–3697. doi: 10.1128/jb.176.12.3692-3697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. N., McGlynn P., Ashby M. K., Burgess J. G., Olsen J. D. DNA sequencing and complementation/deletion analysis of the bchA-puf operon region of Rhodobacter sphaeroides: in vivo mapping of the oxygen-regulated puf promoter. Mol Microbiol. 1991 Nov;5(11):2649–2661. doi: 10.1111/j.1365-2958.1991.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Hunter C. N., van Grondelle R., Olsen J. D. Photosynthetic antenna proteins: 100 ps before photochemistry starts. Trends Biochem Sci. 1989 Feb;14(2):72–76. doi: 10.1016/0968-0004(89)90047-9. [DOI] [PubMed] [Google Scholar]
- Jones M. R., Fowler G. J., Gibson L. C., Grief G. G., Olsen J. D., Crielaard W., Hunter C. N. Mutants of Rhodobacter sphaeroides lacking one or more pigment-protein complexes and complementation with reaction-centre, LH1, and LH2 genes. Mol Microbiol. 1992 May;6(9):1173–1184. doi: 10.1111/j.1365-2958.1992.tb01556.x. [DOI] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides light-harvesting B800-850-alpha and B800-850-beta genes. J Bacteriol. 1987 Jul;169(7):3268–3275. doi: 10.1128/jb.169.7.3268-3275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Klug G. Regulation of expression of photosynthesis genes in anoxygenic photosynthetic bacteria. Arch Microbiol. 1993;159(5):397–404. doi: 10.1007/BF00288584. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Suzue G., Subbarayan C., Porter J. W. The conversion of phytoene-14C to acyclic, monocyclic, and dicyclic carotenes and the conversion of lycopene-15,15'-3H to mono- and dicyclic carotenes by soluble enzyme systems obtained from plastids of tomato fruits. J Biol Chem. 1970 Sep 25;245(18):4708–4717. [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lang H. P., Hunter C. N. The relationship between carotenoid biosynthesis and the assembly of the light-harvesting LH2 complex in Rhodobacter sphaeroides. Biochem J. 1994 Feb 15;298(Pt 1):197–205. doi: 10.1042/bj2980197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. K., Kaplan S. cis-acting regulatory elements involved in oxygen and light control of puc operon transcription in Rhodobacter sphaeroides. J Bacteriol. 1992 Feb;174(4):1146–1157. doi: 10.1128/jb.174.4.1146-1157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H., Misawa N., Chamovitz D., Pecker I., Hirschberg J., Sandmann G. Functional complementation in Escherichia coli of different phytoene desaturase genes and analysis of accumulated carotenes. Z Naturforsch C. 1991 Nov-Dec;46(11-12):1045–1051. doi: 10.1515/znc-1991-11-1219. [DOI] [PubMed] [Google Scholar]
- Martínez-Férez I. M., Vioque A. Nucleotide sequence of the phytoene desaturase gene from Synechocystis sp. PCC 6803 and characterization of a new mutation which confers resistance to the herbicide norflurazon. Plant Mol Biol. 1992 Mar;18(5):981–983. doi: 10.1007/BF00019213. [DOI] [PubMed] [Google Scholar]
- Math S. K., Hearst J. E., Poulter C. D. The crtE gene in Erwinia herbicola encodes geranylgeranyl diphosphate synthase. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6761–6764. doi: 10.1073/pnas.89.15.6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn P., Hunter C. N. Isolation and characterization of a putative transcription factor involved in the regulation of the Rhodobacter sphaeroides pucBA operon. J Biol Chem. 1992 Jun 5;267(16):11098–11103. [PubMed] [Google Scholar]
- Pecker I., Chamovitz D., Linden H., Sandmann G., Hirschberg J. A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4962–4966. doi: 10.1073/pnas.89.11.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sandmann G., Kowalczyk S. In vitro carotenogenesis and characterization of the phytoene desaturase reaction in Anacystis. Biochem Biophys Res Commun. 1989 Sep 15;163(2):916–921. doi: 10.1016/0006-291x(89)92309-7. [DOI] [PubMed] [Google Scholar]
- Sandmann G., Misawa N. New functional assignment of the carotenogenic genes crtB and crtE with constructs of these genes from Erwinia species. FEMS Microbiol Lett. 1992 Jan 15;69(3):253–257. doi: 10.1016/0378-1097(92)90656-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Hearst J. E. Genetic-physical mapping of a photosynthetic gene cluster from R. capsulata. Cell. 1984 Jul;37(3):937–947. doi: 10.1016/0092-8674(84)90428-8. [DOI] [PubMed] [Google Scholar]
- de Bont J. A., Scholten A., Hansen T. A. Dna-Dna hybridization of Rhodopseudomonas capsulata, Rhodopseudomonas sphaeroides and Rhodopseudomonas sulfidophila strains. Arch Microbiol. 1981 Jan;128(3):271–274. doi: 10.1007/BF00422528. [DOI] [PubMed] [Google Scholar]