Abstract
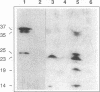
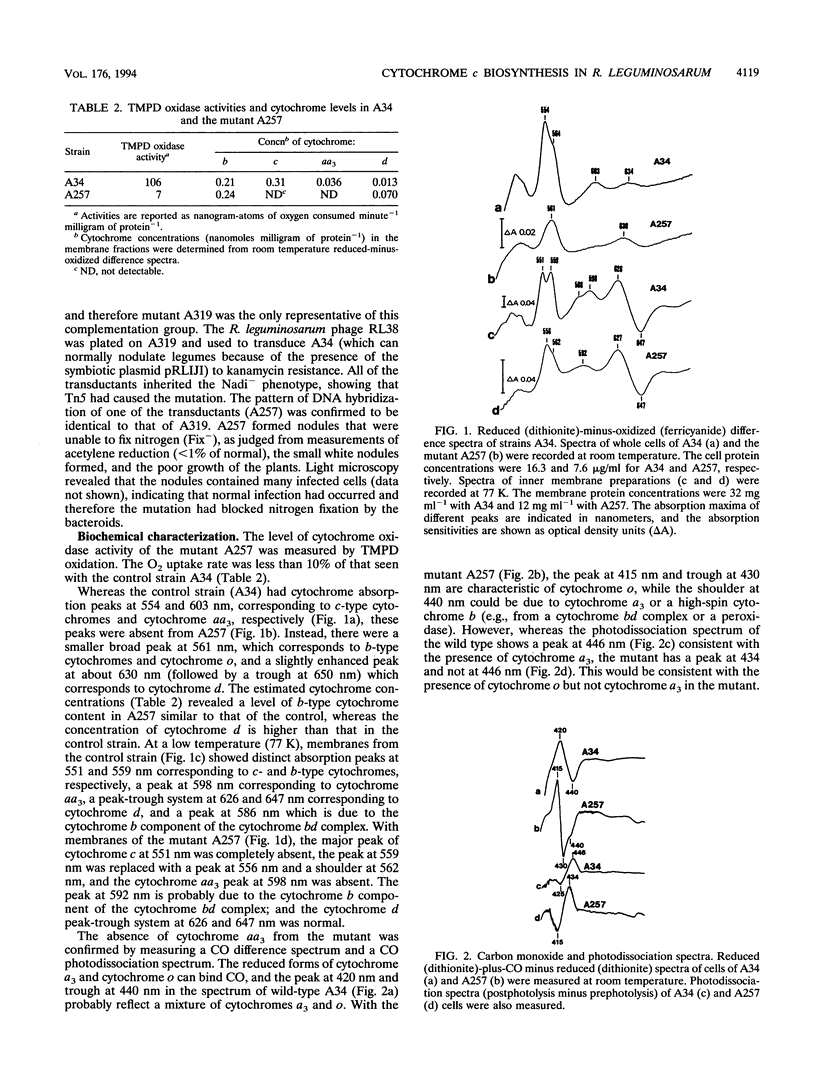
A Tn5-induced mutant of Rhizobium leguminosarum bv. viciae could not form nitrogen-fixing nodules on pea or vetch because of a lesion in electron transport to oxygen. The mutant lacked spectroscopically detectable cytochromes c and aa3. No proteins containing c-type cytochrome could be identified in the mutant by heme staining of proteins fractionated on polyacrylamide gels, indicating that the mutant was defective in maturation of all c-type cytochromes. The Tn5 mutation was determined to be located in a gene that was called cycY. The cycY gene product is homologous to the thioredoxin-like protein HelX involved in the assembly of c-type cytochromes in Rhodobacter capsulatus and to an open reading frame from a Bradyrhizobium japonicum gene cluster containing other genes involved in cytochrome c biogenesis. Our observations are consistent with CycY functioning as a thioredoxin that reduces cysteine residues in apocytochromes c before heme attachment.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. Sequence variability in bacterial cytochromes c. Biochim Biophys Acta. 1991 May 23;1058(1):42–47. doi: 10.1016/s0005-2728(05)80266-x. [DOI] [PubMed] [Google Scholar]
- Appleby C. A., James P., Hennecke H. Characterization of three soluble c-type cytochromes isolated from soybean root nodule bacteroids of Bradyrhizobium japonicum strain CC705. FEMS Microbiol Lett. 1991 Oct 1;67(2):137–144. doi: 10.1016/0378-1097(91)90344-a. [DOI] [PubMed] [Google Scholar]
- Beckman D. L., Kranz R. G. Cytochromes c biogenesis in a photosynthetic bacterium requires a periplasmic thioredoxin-like protein. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2179–2183. doi: 10.1073/pnas.90.6.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman D. L., Trawick D. R., Kranz R. G. Bacterial cytochromes c biogenesis. Genes Dev. 1992 Feb;6(2):268–283. doi: 10.1101/gad.6.2.268. [DOI] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bott M., Ritz D., Hennecke H. The Bradyrhizobium japonicum cycM gene encodes a membrane-anchored homolog of mitochondrial cytochrome c. J Bacteriol. 1991 Nov;173(21):6766–6772. doi: 10.1128/jb.173.21.6766-6772.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. Y., Zhang J. J., Paulus H. Chromosomal location of the Bacillus subtilis aspartokinase II gene and nucleotide sequence of the adjacent genes homologous to uvrC and trx of Escherichia coli. J Gen Microbiol. 1989 Nov;135(11):2931–2940. doi: 10.1099/00221287-135-11-2931. [DOI] [PubMed] [Google Scholar]
- Clement-Metral J. D., Holmgren A., Cambillau C., Jörnvall H., Eklund H., Thomas D., Lederer F. Amino acid sequence determination and three-dimensional modelling of thioredoxin from the photosynthetic bacterium Rhodobacter sphaeroides Y. Eur J Biochem. 1988 Mar 1;172(2):413–419. doi: 10.1111/j.1432-1033.1988.tb13902.x. [DOI] [PubMed] [Google Scholar]
- Colleran E. M., Jones O. T. Studies on the biosynthesis of cytochrome c. Biochem J. 1973 May;134(1):89–96. doi: 10.1042/bj1340089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci J. M., O'Brian M. R. Characterization of a Bradyrhizobium japonicum ferrochelatase mutant and isolation of the hemH gene. J Bacteriol. 1992 Jul;174(13):4223–4229. doi: 10.1128/jb.174.13.4223-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Knight C. D., Rossen L., Robertson J. G., Wells B., Downie J. A. Nodulation inhibition by Rhizobium leguminosarum multicopy nodABC genes and analysis of early stages of plant infection. J Bacteriol. 1986 May;166(2):552–558. doi: 10.1128/jb.166.2.552-558.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R. G. Isolation of mutants and genes involved in cytochromes c biosynthesis in Rhodobacter capsulatus. J Bacteriol. 1989 Jan;171(1):456–464. doi: 10.1128/jb.171.1.456-464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labes M., Pühler A., Simon R. A new family of RSF1010-derived expression and lac-fusion broad-host-range vectors for gram-negative bacteria. Gene. 1990 Apr 30;89(1):37–46. doi: 10.1016/0378-1119(90)90203-4. [DOI] [PubMed] [Google Scholar]
- Lim C. J., Geraghty D., Fuchs J. A. Cloning and nucleotide sequence of the trxA gene of Escherichia coli K-12. J Bacteriol. 1985 Jul;163(1):311–316. doi: 10.1128/jb.163.1.311-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loferer H., Bott M., Hennecke H. Bradyrhizobium japonicum TlpA, a novel membrane-anchored thioredoxin-like protein involved in the biogenesis of cytochrome aa3 and development of symbiosis. EMBO J. 1993 Sep;12(9):3373–3383. doi: 10.1002/j.1460-2075.1993.tb06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Chamberlain S. H., Chu C., Masiarz F. R., Shin S., Yee B. C., Buchanan B. B. Plant thioredoxin h: an animal-like thioredoxin occurring in multiple cell compartments. Arch Biochem Biophys. 1991 May 15;287(1):195–198. doi: 10.1016/0003-9861(91)90406-9. [DOI] [PubMed] [Google Scholar]
- Marrs B., Gest H. Genetic mutations affecting the respiratory electron-transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1973 Jun;114(3):1045–1051. doi: 10.1128/jb.114.3.1045-1051.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty I., Meyer Y. Nucleotide sequence of a cDNA encoding a tobacco thioredoxin. Plant Mol Biol. 1991 Jul;17(1):143–147. doi: 10.1007/BF00036817. [DOI] [PubMed] [Google Scholar]
- McFarlan S. C., Hogenkamp H. P., Eccleston E. D., Howard J. B., Fuchs J. A. Purification, characterization and revised amino acid sequence of a second thioredoxin from Corynebacterium nephridii. Eur J Biochem. 1989 Feb 1;179(2):389–398. doi: 10.1111/j.1432-1033.1989.tb14565.x. [DOI] [PubMed] [Google Scholar]
- O'Brian M. R., Kirshbom P. M., Maier R. J. Bacterial heme synthesis is required for expression of the leghemoglobin holoprotein but not the apoprotein in soybean root nodules. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8390–8393. doi: 10.1073/pnas.84.23.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. D., Ferguson S. J. Apo forms of cytochrome c550 and cytochrome cd1 are translocated to the periplasm of Paracoccus denitrificans in the absence of haem incorporation caused either mutation or inhibition of haem synthesis. Mol Microbiol. 1990 Jul;4(7):1181–1192. doi: 10.1111/j.1365-2958.1990.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Ramseier T. M., Winteler H. V., Hennecke H. Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J Biol Chem. 1991 Apr 25;266(12):7793–7803. [PubMed] [Google Scholar]
- Ritz D., Bott M., Hennecke H. Formation of several bacterial c-type cytochromes requires a novel membrane-anchored protein that faces the periplasm. Mol Microbiol. 1993 Aug;9(4):729–740. doi: 10.1111/j.1365-2958.1993.tb01733.x. [DOI] [PubMed] [Google Scholar]
- Rossbach S., Loferer H., Acuña G., Appleby C. A., Hennecke H. Cloning, sequencing and mutational analysis of the cytochrome c552 gene (cycB) from Bradyrhizobium japonicum strain 110. FEMS Microbiol Lett. 1991 Oct 1;67(2):145–152. doi: 10.1016/0378-1097(91)90345-b. [DOI] [PubMed] [Google Scholar]
- Soberón M., Aguilar G. R., Sánchez F. Rhizobium phaseoli cytochrome c-deficient mutant induces empty nodules on Phaseolus vulgaris L. Mol Microbiol. 1993 Apr;8(1):159–166. doi: 10.1111/j.1365-2958.1993.tb01212.x. [DOI] [PubMed] [Google Scholar]
- Soberón M., Williams H. D., Poole R. K., Escamilla E. Isolation of a Rhizobium phaseoli cytochrome mutant with enhanced respiration and symbiotic nitrogen fixation. J Bacteriol. 1989 Jan;171(1):465–472. doi: 10.1128/jb.171.1.465-472.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöny-Meyer L., Stax D., Hennecke H. An unusual gene cluster for the cytochrome bc1 complex in Bradyrhizobium japonicum and its requirement for effective root nodule symbiosis. Cell. 1989 May 19;57(4):683–697. doi: 10.1016/0092-8674(89)90137-2. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Sadowsky M. J., Keister D. L. Characterization of cytochromes c550 and c555 from Bradyrhizobium japonicum: cloning, mutagenesis, and sequencing of the c555 gene (cycC). J Bacteriol. 1991 Dec;173(24):7887–7895. doi: 10.1128/jb.173.24.7887-7895.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas C., McEwan A. G., Downie J. A. Detection of c-type cytochromes using enhanced chemiluminescence. Anal Biochem. 1993 Mar;209(2):323–326. doi: 10.1006/abio.1993.1127. [DOI] [PubMed] [Google Scholar]