Abstract
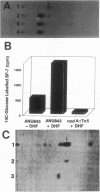
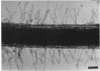
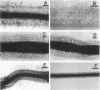
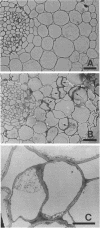
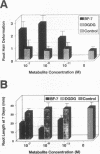
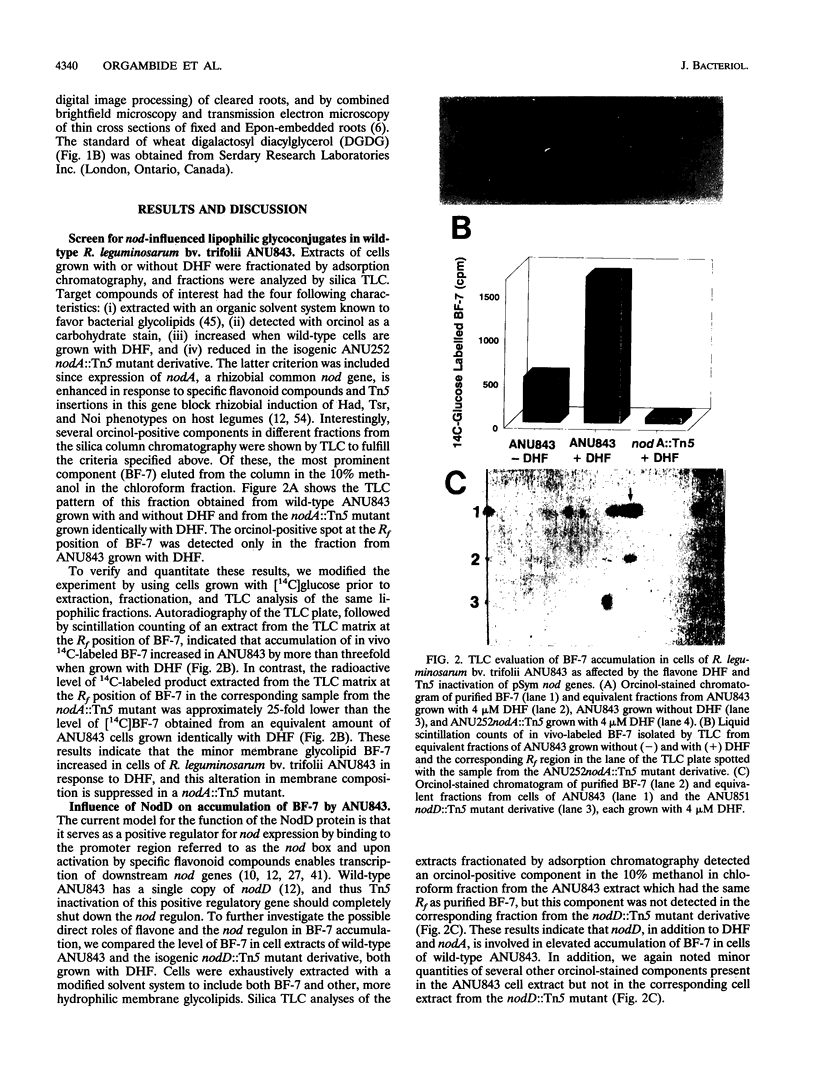
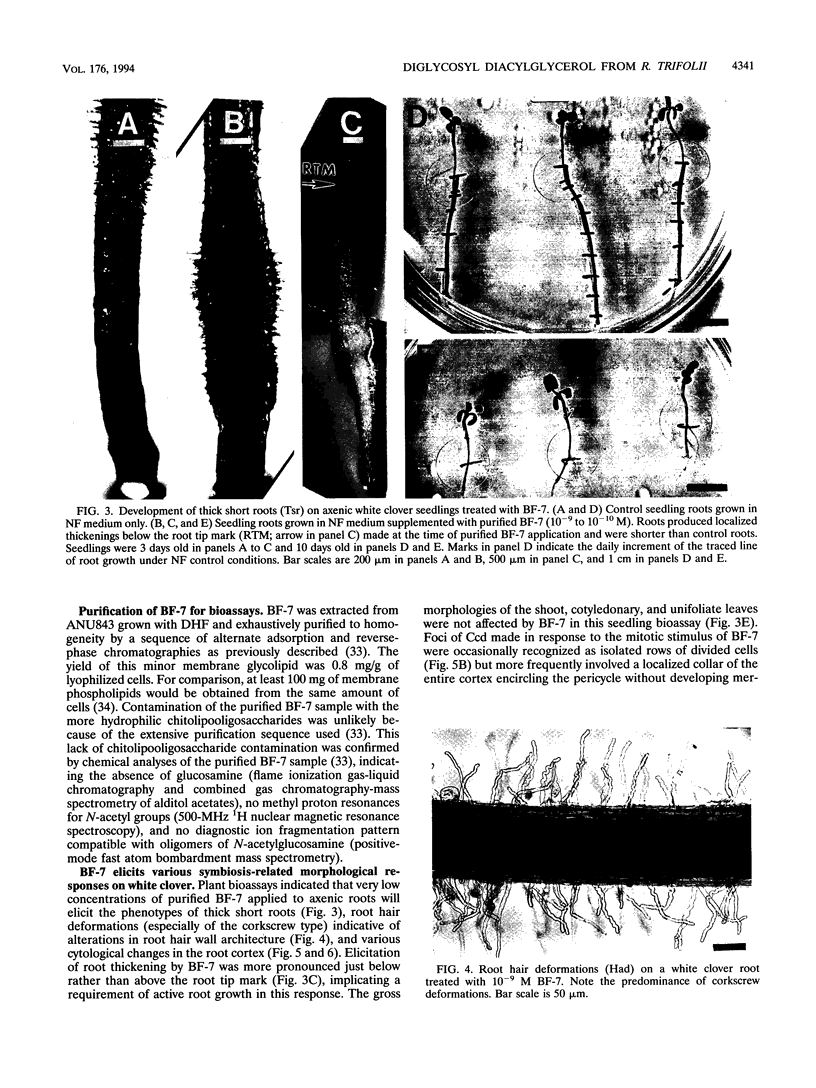
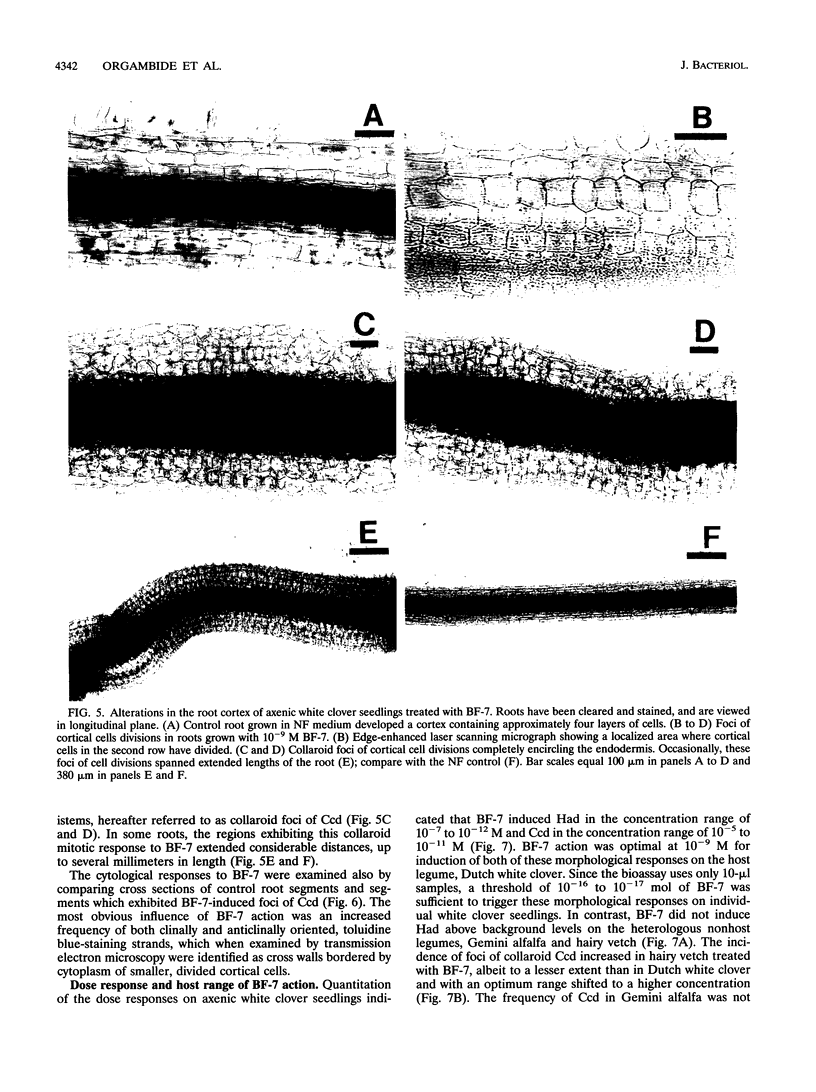
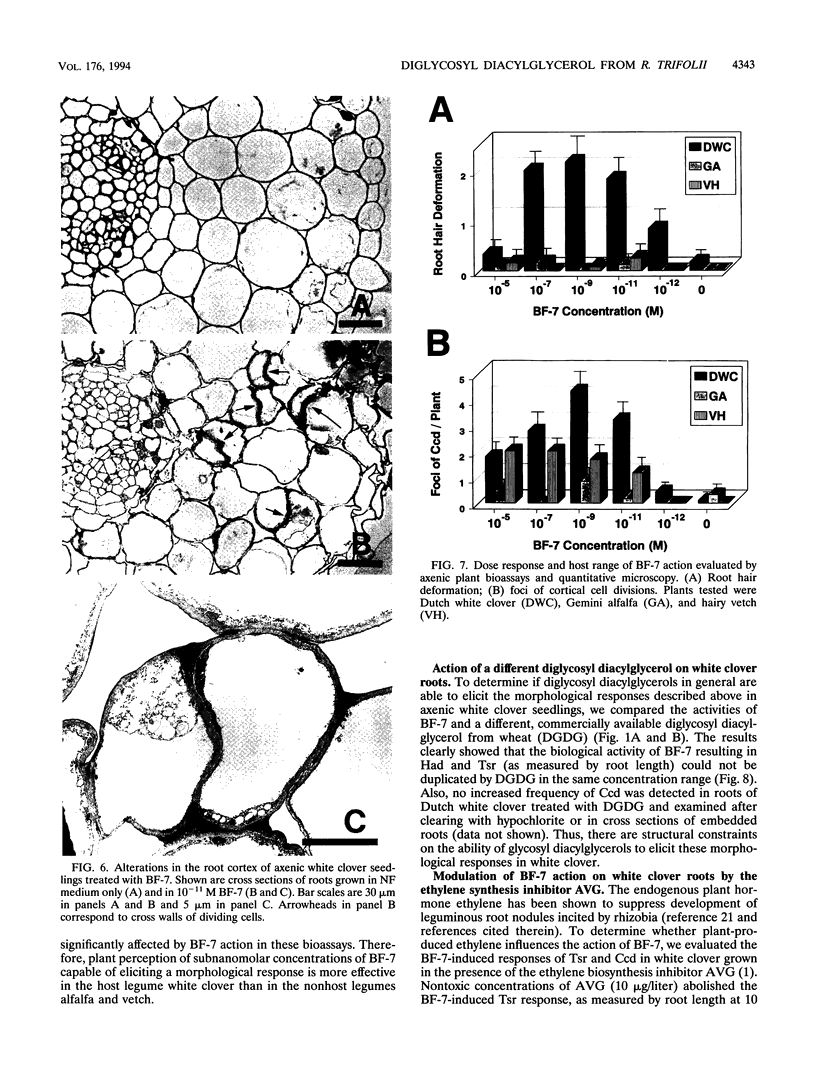
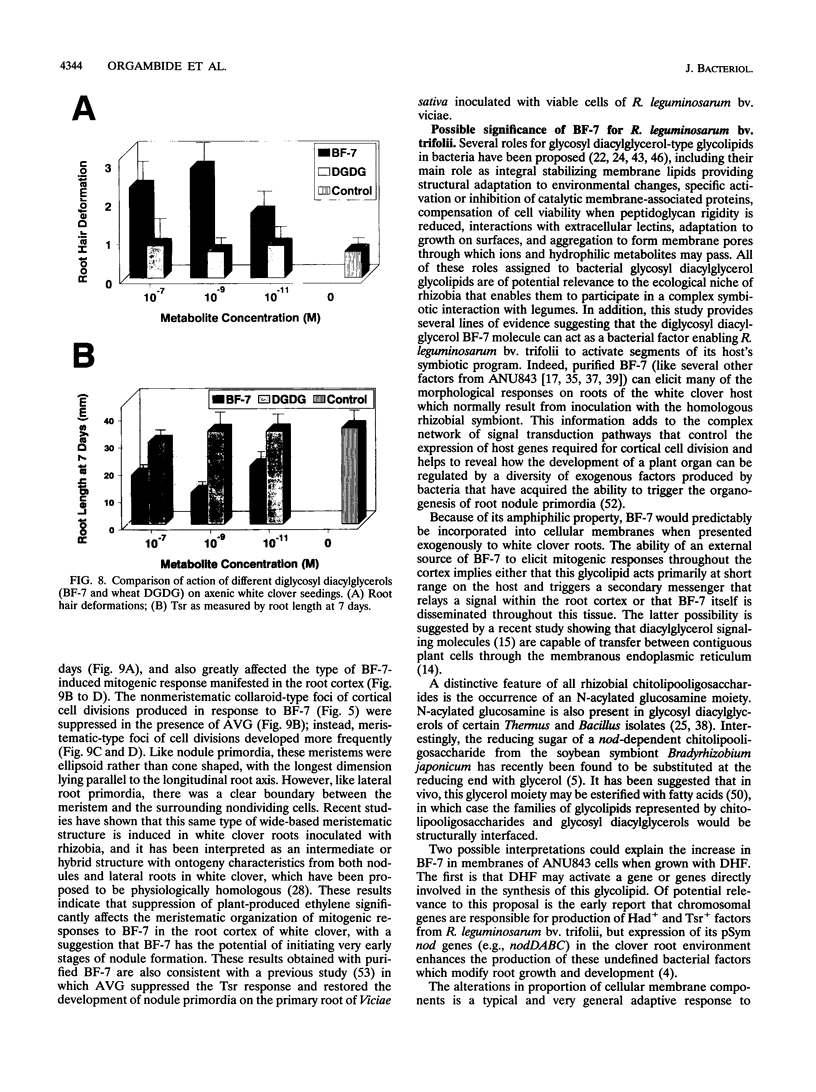
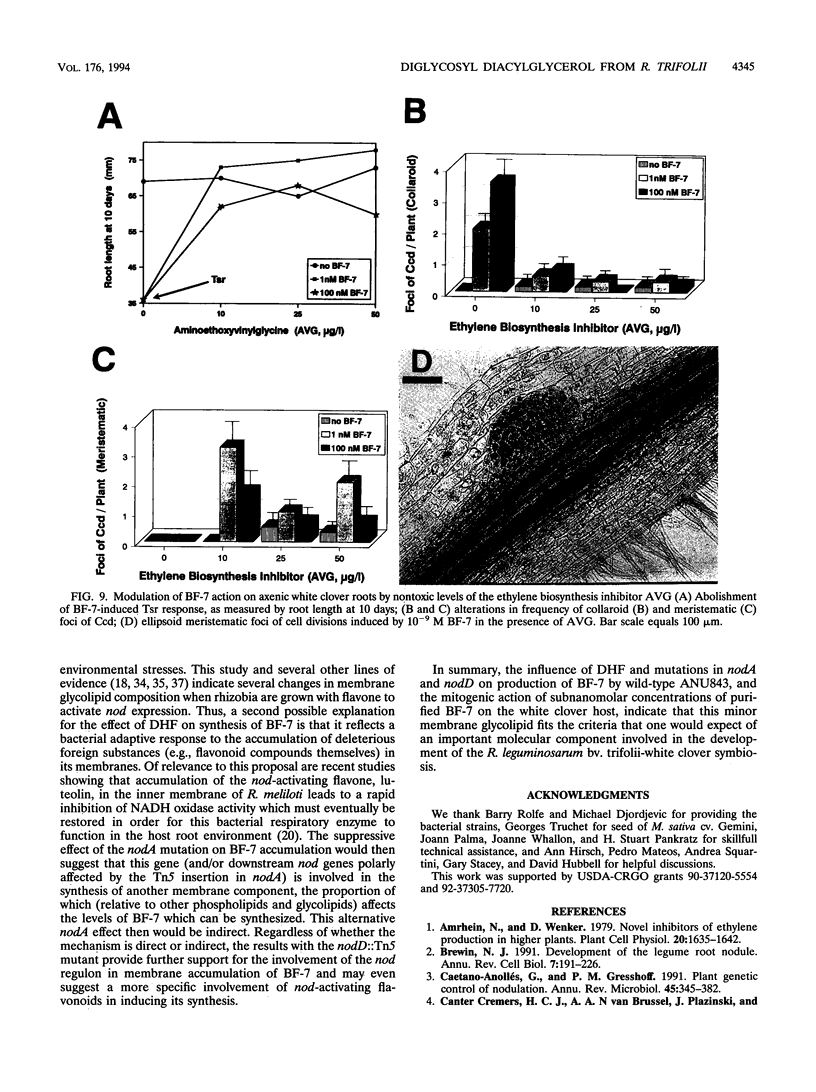
Rhizobium leguminosarum bv. trifolii is the bacterial symbiont which induces nitrogen-fixing root nodules on the leguminous host, white clover (Trifolium repens L.). In this plant-microbe interaction, the host plant excretes a flavone, 4',7-dihydroxyflavone (DHF), which activates expression of modulation genes, enabling the bacterial symbiont to elicit various symbiosis-related morphological changes in its roots. We have investigated the accumulation of a diglycosyl diacylglycerol (BF-7) in wild-type R. leguminosarum bv. trifolii ANU843 when grown with DHF and the biological activities of this glycolipid bacterial factor on host and nonhost legumes. In vivo labeling studies indicated that wild-type ANU843 cells accumulate BF-7 in response to DHF, and this flavone-enhanced alteration in membrane glycolipid composition was suppressed in isogenic nodA::Tn5 and nodD::Tn5 mutant derivatives. Seedling bioassays performed under microbiologically controlled conditions indicated that subnanomolar concentrations of purified BF-7 elicit various symbiosis-related morphological responses on white clover roots, including thick short roots, root hair deformation, and foci of cortical cell divisions. Roots of the nonhost legumes alfalfa and vetch were much less responsive to BF-7 at these low concentrations. A structurally distinct diglycosyl diacylglycerol did not induce these responses on white clover, indicating structural constraints in the biological activity of BF-7 on this legume host. In bioassays using aminoethoxyvinylglycine to suppress plant production of ethylene, BF-7 elicited a meristematic rather than collaroid type of mitogenic response in the root cortex of white clover. These results indicate an involvement of flavone-activated nod expression in membrane accumulation of BF-7 and a potent ability of this diglycosyl diacylglycerol glycolipid to perform as a bacterial factor enabling R. leguminosarum bv. trifolii to activate segments of its host's symbiotic program during early development of the root nodule symbiosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewin N. J. Development of the legume root nodule. Annu Rev Cell Biol. 1991;7:191–226. doi: 10.1146/annurev.cb.07.110191.001203. [DOI] [PubMed] [Google Scholar]
- Caetano-Anollés G., Gresshoff P. M. Plant genetic control of nodulation. Annu Rev Microbiol. 1991;45:345–382. doi: 10.1146/annurev.mi.45.100191.002021. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Sanjuan J., Bhat U. R., Glushka J., Spaink H. P., Wijfjes A. H., van Brussel A. A., Stokkermans T. J., Peters N. K., Stacey G. The structures and biological activities of the lipo-oligosaccharide nodulation signals produced by type I and II strains of Bradyrhizobium japonicum. J Biol Chem. 1993 Aug 25;268(24):18372–18381. [PubMed] [Google Scholar]
- Carlson R. W., Sanjuan J., Bhat U. R., Glushka J., Spaink H. P., Wijfjes A. H., van Brussel A. A., Stokkermans T. J., Peters N. K., Stacey G. The structures and biological activities of the lipo-oligosaccharide nodulation signals produced by type I and II strains of Bradyrhizobium japonicum. J Biol Chem. 1993 Aug 25;268(24):18372–18381. [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Hollingsworth R. I., Hrabak E. M., Pankratz H. S., Philip-Hollingsworth S., Salzwedel J. L., Chapman K., Appenzeller L., Squartini A. Rhizobium lipopolysaccharide modulates infection thread development in white clover root hairs. J Bacteriol. 1991 Sep;173(17):5371–5384. doi: 10.1128/jb.173.17.5371-5384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dénarié J., Debellé F., Rosenberg C. Signaling and host range variation in nodulation. Annu Rev Microbiol. 1992;46:497–531. doi: 10.1146/annurev.mi.46.100192.002433. [DOI] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Grabski S., De Feijter A. W., Schindler M. Endoplasmic Reticulum Forms a Dynamic Continuum for Lipid Diffusion between Contiguous Soybean Root Cells. Plant Cell. 1993 Jan;5(1):25–38. doi: 10.1105/tpc.5.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hrabak E. M., Urbano M. R., Dazzo F. B. Growth-phase-dependent immunodeterminants of Rhizobium trifolii lipopolysaccharide which bind trifoliin A, a white clover lectin. J Bacteriol. 1981 Nov;148(2):697–711. doi: 10.1128/jb.148.2.697-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter W. J. Ethylene Production by Root Nodules and Effect of Ethylene on Nodulation in Glycine max. Appl Environ Microbiol. 1993 Jun;59(6):1947–1950. doi: 10.1128/aem.59.6.1947-1950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langworthy T. A., Mayberry W. R., Smith P. F. A sulfonolipid and novel glucosamidyl glycolipids from the extreme thermoacidophile Bacillus acidocaldarius. Biochim Biophys Acta. 1976 Jun 22;431(3):550–569. doi: 10.1016/0005-2760(76)90220-4. [DOI] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- McKay I. A., Djordjevic M. A. Production and Excretion of Nod Metabolites by Rhizobium leguminosarum bv. trifolii Are Disrupted by the Same Environmental Factors That Reduce Nodulation in the Field. Appl Environ Microbiol. 1993 Oct;59(10):3385–3392. doi: 10.1128/aem.59.10.3385-3392.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgambide G. G., Hollingsworth R. I., Dazzo F. B. Structural characterization of a novel diglycosyl diacylglyceride glycolipid from Rhizobium trifolii ANU843. Carbohydr Res. 1992 Sep 2;233:151–159. doi: 10.1016/s0008-6215(00)90927-3. [DOI] [PubMed] [Google Scholar]
- Orgambide G. G., Huang Z. H., Gage D. A., Dazzo F. B. Phospholipid and fatty acid compositions of Rhizobium leguminosarum biovar trifolii ANU843 in relation to flavone-activated pSym nod gene expression. Lipids. 1993 Nov;28(11):975–979. doi: 10.1007/BF02537117. [DOI] [PubMed] [Google Scholar]
- Orgambide G., Philip-Hollingsworth S., Cargill L., Dazzo F. Evaluation of acidic heteropolysaccharide structures in Rhizobium leguminosarum biovars altered in nodulation genes and host range. Mol Plant Microbe Interact. 1992 Nov-Dec;5(6):484–488. doi: 10.1094/mpmi-5-484. [DOI] [PubMed] [Google Scholar]
- Pask-Hughes R. A., Shaw N. Glycolipids from some extreme thermophilic bacteria belonging to the genus Thermus. J Bacteriol. 1982 Jan;149(1):54–58. doi: 10.1128/jb.149.1.54-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip-Hollingsworth S., Hollingsworth R. I., Dazzo F. B. N-Acetylglutamic acid: an extracellular nod signal of Rhizobium trifolii ANU843 that induces root hair branching and nodule-like primordia in white clover roots. J Biol Chem. 1991 Sep 5;266(25):16854–16858. [PubMed] [Google Scholar]
- Philip-Hollingsworth S., Hollingsworth R. I., Dazzo F. B. N-Acetylglutamic acid: an extracellular nod signal of Rhizobium trifolii ANU843 that induces root hair branching and nodule-like primordia in white clover roots. J Biol Chem. 1991 Sep 5;266(25):16854–16858. [PubMed] [Google Scholar]
- Price N. P., Relić B., Talmont F., Lewin A., Promé D., Pueppke S. G., Maillet F., Dénarié J., Promé J. C., Broughton W. J. Broad-host-range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulphated. Mol Microbiol. 1992 Dec;6(23):3575–3584. doi: 10.1111/j.1365-2958.1992.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Sastry P. S. Glycosyl glycerides. Adv Lipid Res. 1974;12(0):251–310. doi: 10.1016/b978-0-12-024912-1.50013-2. [DOI] [PubMed] [Google Scholar]
- Schultze M., Quiclet-Sire B., Kondorosi E., Virelizer H., Glushka J. N., Endre G., Géro S. D., Kondorosi A. Rhizobium meliloti produces a family of sulfated lipooligosaccharides exhibiting different degrees of plant host specificity. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):192–196. doi: 10.1073/pnas.89.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw N. Bacterial glycolipids. Bacteriol Rev. 1970 Dec;34(4):365–377. doi: 10.1128/br.34.4.365-377.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Liau Y. H., Slomiany A. Animal glycoglycerolipids. Prog Lipid Res. 1987;26(1):29–51. doi: 10.1016/0163-7827(87)90007-5. [DOI] [PubMed] [Google Scholar]
- Spaink H. P. Rhizobial lipo-oligosaccharides: answers and questions. Plant Mol Biol. 1992 Dec;20(5):977–986. doi: 10.1007/BF00027167. [DOI] [PubMed] [Google Scholar]
- Spaink H. P., Sheeley D. M., van Brussel A. A., Glushka J., York W. S., Tak T., Geiger O., Kennedy E. P., Reinhold V. N., Lugtenberg B. J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991 Nov 14;354(6349):125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Verma DPS. Signals in Root Nodule Organogenesis and Endocytosis of Rhizobium. Plant Cell. 1992 Apr;4(4):373–382. doi: 10.1105/tpc.4.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat S. A., van Brussel A. A., Tak T., Pees E., Lugtenberg B. J. Flavonoids induce Rhizobium leguminosarum to produce nodDABC gene-related factors that cause thick, short roots and root hair responses on common vetch. J Bacteriol. 1987 Jul;169(7):3388–3391. doi: 10.1128/jb.169.7.3388-3391.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]