Abstract
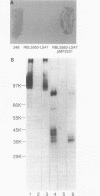
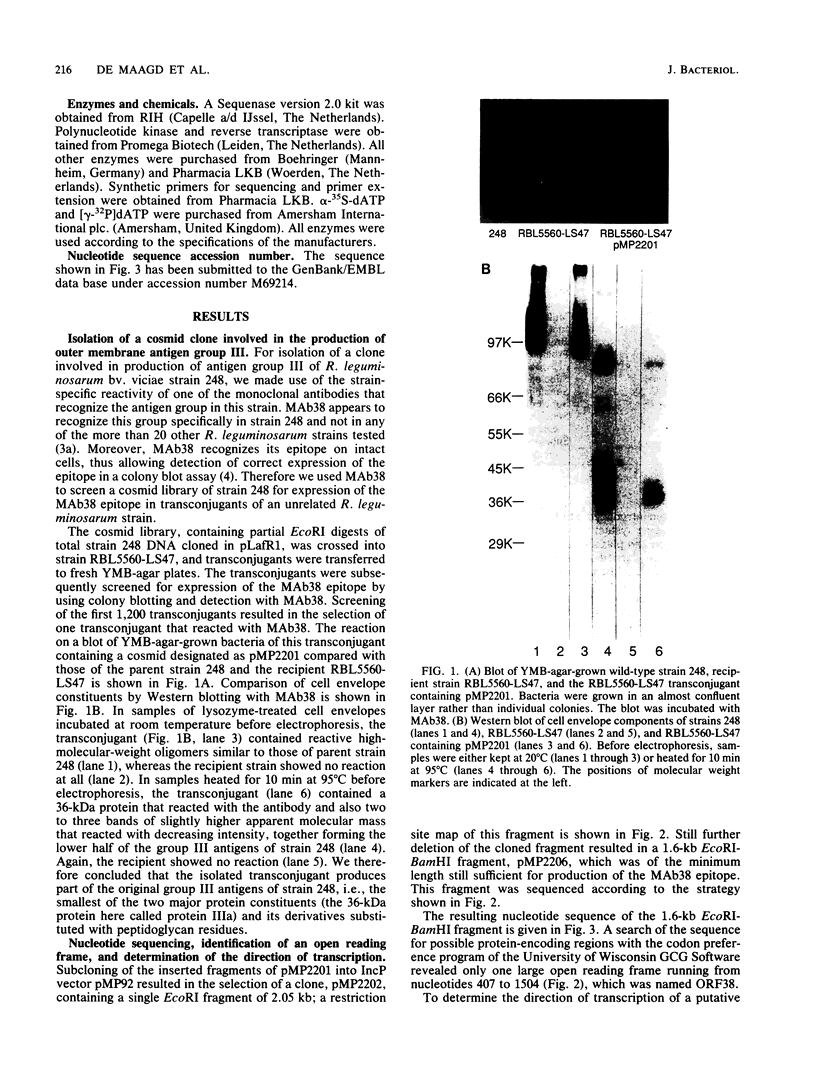
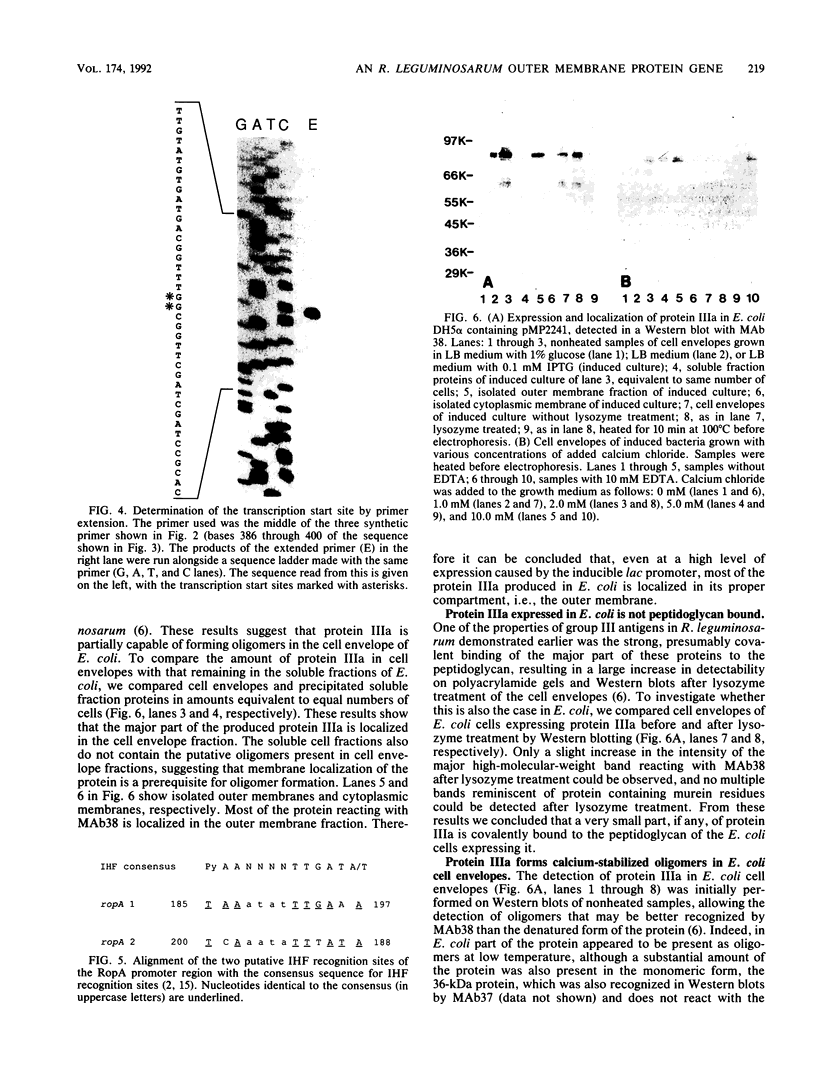
We describe the cloning of a gene from Rhizobium leguminosarum biovar viciae strain 248 encoding protein IIIa, the 36-kDa outer membrane protein forming a part of the outer membrane protein antigen group III. The expression of this antigen group is repressed in the bacteroid form during symbiosis (R. A. de Maagd, R. de Rijk, I. H. M. Mulders, and B. J. J. Lugtenberg, J. Bacteriol. 171:1136-1142, 1989). A cosmid clone expressing the strain 248-specific MAb38 epitope of this antigen group in a nonrelated strain was selected by a colony blot assay. Sequencing revealed one large open reading frame encoding a 39-kDa protein. N-terminal amino acid sequencing of the purified 36-kDa outer membrane protein IIIa revealed that the isolated gene, now designated ropA, is the structural gene for this protein and that the mature protein was formed by processing of the 22-residue N-terminal signal sequence. The gene is preceded by a promoter that was active in R. leguminosarum but not in Escherichia coli. This promoter, which showed no homology to known promoter sequences, was located approximately by determination of the transcription start site. The region upstream of the putative promoter was shown to contain two potential binding sites for integration host factor protein. Expression of protein IIIa under control of the inducible lac promoter in E. coli shows that, of its earlier described properties, the peptidoglycan linkage of protein IIIa is specific for R. leguminosarum but that outer membrane localization and calcium-stabilized oligomer formation can to a large extent also occur in E. coli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Goosen-de Roo L., de Maagd R. A., Lugtenberg B. J. Antigenic changes in lipopolysaccharide I of Rhizobium leguminosarum bv. viciae in root nodules of Vicia sativa subsp. nigra occur during release from infection threads. J Bacteriol. 1991 May;173(10):3177–3183. doi: 10.1128/jb.173.10.3177-3183.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F. G., Dahl H. H., de Boer E., Flavell R. A. Isolation of beta-globin-related genes from a human cosmid library. Gene. 1981 Apr;13(3):227–237. doi: 10.1016/0378-1119(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Hoover T. R., Santero E., Porter S., Kustu S. The integration host factor stimulates interaction of RNA polymerase with NIFA, the transcriptional activator for nitrogen fixation operons. Cell. 1990 Oct 5;63(1):11–22. doi: 10.1016/0092-8674(90)90284-l. [DOI] [PubMed] [Google Scholar]
- Hooykaas P. J., Snijdewint F. G., Schilperoort R. A. Identification of the Sym plasmid of Rhizobium leguminosarum strain 1001 and its transfer to and expression in other rhizobia and Agrobacterium tumefaciens. Plasmid. 1982 Jul;8(1):73–82. doi: 10.1016/0147-619x(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Lecocq J. P. New versatile cloning and sequencing vectors based on bacteriophage M13. Gene. 1983 Dec;26(1):91–99. doi: 10.1016/0378-1119(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Leong J. M., Nunes-Düby S., Lesser C. F., Youderian P., Susskind M. M., Landy A. The phi 80 and P22 attachment sites. Primary structure and interaction with Escherichia coli integration host factor. J Biol Chem. 1985 Apr 10;260(7):4468–4477. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu S. S., Brewin N. J., Kannenberg E. L. Immunochemical analysis of lipopolysaccharides from free-living and endosymbiotic forms of Rhizobium leguminosarum. J Bacteriol. 1990 Apr;172(4):1804–1813. doi: 10.1128/jb.172.4.1804-1813.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyvé M., Moons M., Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991 Mar 5;218(1):141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- VandenBosch K. A., Brewin N. J., Kannenberg E. L. Developmental regulation of a Rhizobium cell surface antigen during growth of pea root nodules. J Bacteriol. 1989 Sep;171(9):4537–4542. doi: 10.1128/jb.171.9.4537-4542.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel A., Weiss D. S., Popham D., Dröge P., Kustu S. A bacterial enhancer functions to tether a transcriptional activator near a promoter. Science. 1990 Apr 27;248(4954):486–490. doi: 10.1126/science.1970441. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Maagd R. A., Wientjes F. B., Lugtenberg B. J. Evidence for divalent cation (Ca2+)-stabilized oligomeric proteins and covalently bound protein-peptidoglycan complexes in the outer membrane of Rhizobium leguminosarum. J Bacteriol. 1989 Jul;171(7):3989–3995. doi: 10.1128/jb.171.7.3989-3995.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R., de Rijk R., Mulders I. H., Lugtenberg B. J. Immunological characterization of Rhizobium leguminosarum outer membrane antigens by use of polyclonal and monoclonal antibodies. J Bacteriol. 1989 Feb;171(2):1136–1142. doi: 10.1128/jb.171.2.1136-1142.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R., van Rossum C., Lugtenberg B. J. Recognition of individual strains of fast-growing rhizobia by using profiles of membrane proteins and lipopolysaccharides. J Bacteriol. 1988 Aug;170(8):3782–3785. doi: 10.1128/jb.170.8.3782-3785.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]