Abstract
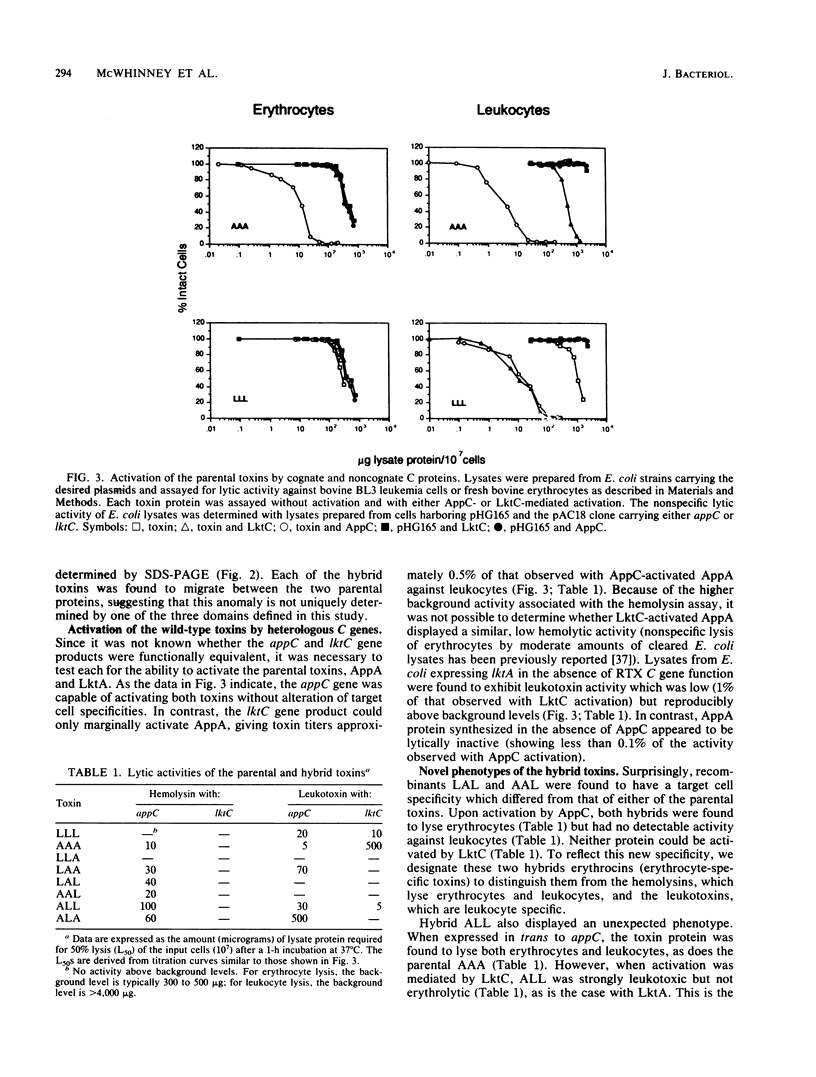
The leukotoxin (LktA) from Pasteurella haemolytica and the hemolysin (AppA) from Actinobacillus pleuropneumoniae are members of a highly conserved family of cytolytic proteins produced by gram-negative bacteria. Despite the extensive homology between these gene products, LktA is specific for ruminant leukocytes while AppA, like other hemolysins, lyses erythrocytes and a variety of nucleated cells, including ruminant leukocytes. Both proteins require activation facilitated by the product of an accessory repeat toxin (RTX) C gene for optimal biological activity. We have constructed six genes encoding hybrid toxins by recombining domains of ltkA and appA and have examined the target cell specificities of the resulting hybrid proteins. Our results indicate that the leukocytic potential of AppA, like that of LktA, maps to the C-terminal half of the protein and is physically separable from the region specifying erythrocyte lysis. As a consequence, we were able to construct an RTX toxin capable of lysing erythrocytes but not leukocytes. The specificity of one hybrid was found to be dependent upon the RTX C gene used for activation. With appC activation, this hybrid toxin lysed both erythrocytes and leukocytes, while lktC activation produced a toxin which could attack only leukocytes. This is the first demonstration that the specificity of an RTX toxin can be determined by the process of C-mediated activation.
Full text
PDF


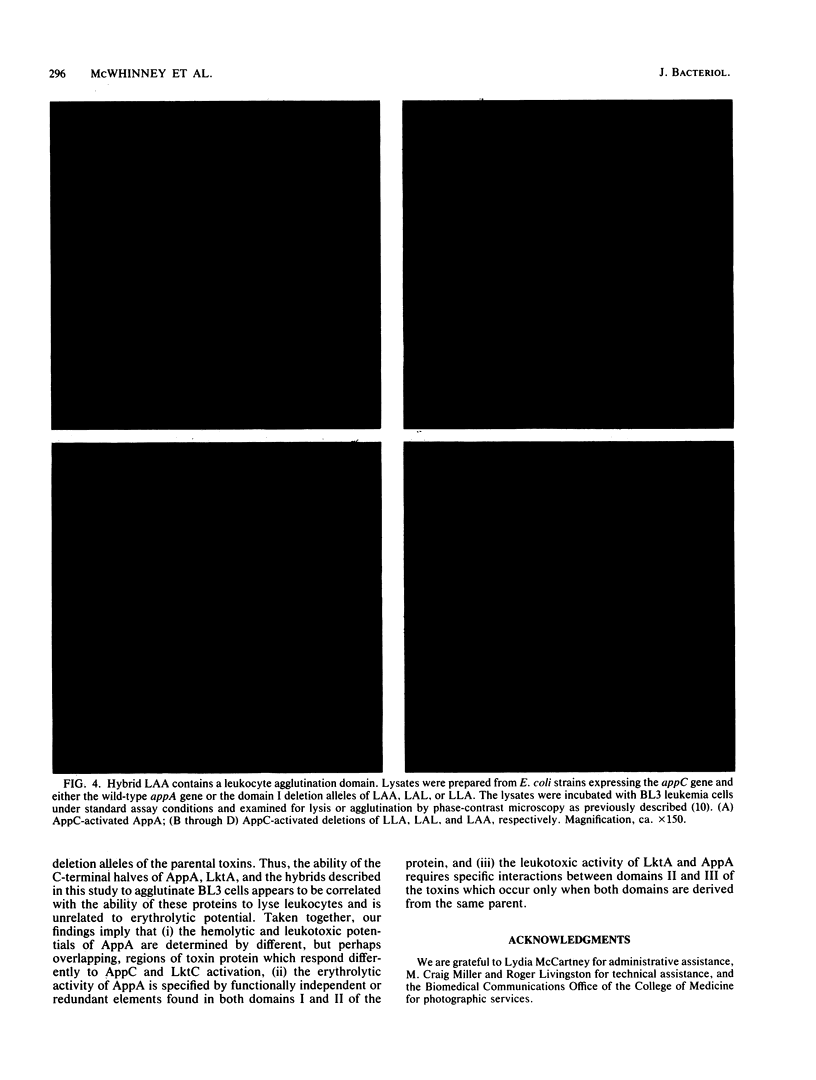

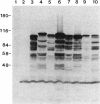
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Altman R. K., Garrett J. M., Grimaila R. J., Young R. S gene product: identification and membrane localization of a lysis control protein. J Bacteriol. 1983 Sep;155(3):1130–1137. doi: 10.1128/jb.155.3.1130-1137.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Moulds T. L., Struck D. K. Secretion of the Pasteurella leukotoxin by Escherichia coli. FEMS Microbiol Lett. 1989 Jul 15;51(1):169–173. doi: 10.1016/0378-1097(89)90502-8. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Post D., Struck D. K. Identification and characterization of the Pasteurella haemolytica leukotoxin. Infect Immun. 1987 Oct;55(10):2348–2354. doi: 10.1128/iai.55.10.2348-2354.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA. 1989 Nov;8(9):635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. The Actinobacillus pleuropneumoniae hemolysin determinant: unlinked appCA and appBD loci flanked by pseudogenes. J Bacteriol. 1991 Aug;173(16):5151–5158. doi: 10.1128/jb.173.16.5151-5158.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz W. T., Young R., Chang Y. F., Struck D. K. Deletion analysis resolves cell-binding and lytic domains of the Pasteurella leukotoxin. Mol Microbiol. 1990 Nov;4(11):1933–1939. doi: 10.1111/j.1365-2958.1990.tb02042.x. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier C., Welch R. A. Nonreciprocal complementation of the hlyC and lktC genes of the Escherichia coli hemolysin and Pasteurella haemolytica leukotoxin determinants. Infect Immun. 1990 Mar;58(3):828–832. doi: 10.1128/iai.58.3.828-832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L., Baker K., Kenny B., Mackman N., Haigh R., Holland I. B. A novel C-terminal signal sequence targets Escherichia coli haemolysin directly to the medium. J Cell Sci Suppl. 1989;11:45–57. doi: 10.1242/jcs.1989.supplement_11.4. [DOI] [PubMed] [Google Scholar]
- Gray L., Mackman N., Nicaud J. M., Holland I. B. The carboxy-terminal region of haemolysin 2001 is required for secretion of the toxin from Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):127–133. doi: 10.1007/BF02428042. [DOI] [PubMed] [Google Scholar]
- Gygi D., Nicolet J., Frey J., Cross M., Koronakis V., Hughes C. Isolation of the Actinobacillus pleuropneumoniae haemolysin gene and the activation and secretion of the prohaemolysin by the HlyC, HlyB and HlyD proteins of Escherichia coli. Mol Microbiol. 1990 Jan;4(1):123–128. doi: 10.1111/j.1365-2958.1990.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Hess J., Gentschev I., Goebel W., Jarchau T. Analysis of the haemolysin secretion system by PhoA-HlyA fusion proteins. Mol Gen Genet. 1990 Nov;224(2):201–208. doi: 10.1007/BF00271553. [DOI] [PubMed] [Google Scholar]
- Highlander S. K., Chidambaram M., Engler M. J., Weinstock G. M. DNA sequence of the Pasteurella haemolytica leukotoxin gene cluster. DNA. 1989 Jan-Feb;8(1):15–28. doi: 10.1089/dna.1.1989.8.15. [DOI] [PubMed] [Google Scholar]
- Highlander S. K., Engler M. J., Weinstock G. M. Secretion and expression of the Pasteurella haemolytica Leukotoxin. J Bacteriol. 1990 May;172(5):2343–2350. doi: 10.1128/jb.172.5.2343-2350.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston T. C., Thompson R. B., Baldwin T. O. Nucleotide sequence of the luxB gene of Vibrio harveyi and the complete amino acid sequence of the beta subunit of bacterial luciferase. J Biol Chem. 1986 Apr 15;261(11):4805–4811. [PubMed] [Google Scholar]
- Koronakis V., Cross M., Senior B., Koronakis E., Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Koronakis E., Hughes C. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 1989 Feb;8(2):595–605. doi: 10.1002/j.1460-2075.1989.tb03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig E., Dailey T., Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: homology to the alpha-hemolysin/leukotoxin gene family. Infect Immun. 1990 Apr;58(4):920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally E. T., Golub E. E., Kieba I. R., Taichman N. S., Rosenbloom J., Rosenbloom J. C., Gibson C. W., Demuth D. R. Analysis of the Actinobacillus actinomycetemcomitans leukotoxin gene. Delineation of unique features and comparison to homologous toxins. J Biol Chem. 1989 Sep 15;264(26):15451–15456. [PubMed] [Google Scholar]
- Lo R. Y., Shewen P. E., Strathdee C. A., Greer C. N. Cloning and expression of the leukotoxin gene of Pasteurella haemolytica A1 in Escherichia coli K-12. Infect Immun. 1985 Dec;50(3):667–671. doi: 10.1128/iai.50.3.667-671.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Strathdee C. A., Shewen P. E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987 Sep;55(9):1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Vogel M., Goebel W. Mutations affecting activity and transport of haemolysin in Escherichia coli. Mol Gen Genet. 1987 Feb;206(2):238–245. doi: 10.1007/BF00333579. [DOI] [PubMed] [Google Scholar]
- Nicaud J. M., Mackman N., Gray L., Holland I. B. Characterisation of HlyC and mechanism of activation and secretion of haemolysin from E. coli 2001. FEBS Lett. 1985 Aug 5;187(2):339–344. doi: 10.1016/0014-5793(85)81272-2. [DOI] [PubMed] [Google Scholar]
- Shewen P. E., Wilkie B. N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982 Jan;35(1):91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. L., Berthold P., Taichman N. S. Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 1988 May;56(5):1162–1166. doi: 10.1128/iai.56.5.1162-1166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. S., Lubinsky-Mink S., Jackson C. G., Cassel A., Kuhn J. pHG165: a pBR322 copy number derivative of pUC8 for cloning and expression. Plasmid. 1986 May;15(3):172–181. doi: 10.1016/0147-619x(86)90035-1. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Regulation of expression of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Nov;171(11):5955–5962. doi: 10.1128/jb.171.11.5955-5962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W., Kuhn M., Goebel W. Active and inactive forms of hemolysin (HlyA) from Escherichia coli. Biol Chem Hoppe Seyler. 1988 Jan;369(1):39–46. doi: 10.1515/bchm3.1988.369.1.39. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]