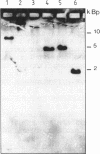
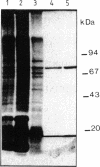
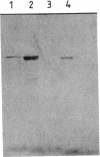
Abstract
The pentacyclic hopanoids, a class of eubacterial lipids, are synthesized by squalene-hopene cyclase and side chain-elongating enzymes. With the aid of DNA probes based on the amino-terminal sequence of purified squalene-hopene cyclase from Bacillus acidocaldarius, clones of Escherichia coli that express this enzyme in the cytoplasmic membrane were isolated. According to the DNA sequence, the cyclase contained 627 amino acids with a molecular mass of 69,473 Da. A high percentage of the amino acids were basic. No significant similarity to existing sequenced proteins was found.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askelöf P., Rodmalm K., Abens J., Undén A., Bartfai T. Use of synthetic peptides to map antigenic sites of Bordetella pertussis toxin subunit S1. J Infect Dis. 1988 Apr;157(4):738–742. doi: 10.1093/infdis/157.4.738. [DOI] [PubMed] [Google Scholar]
- Benz R., Hallmann D., Poralla K., Eibl H. Interaction of hopanoids with phosphatidylcholines containing oleic and omega-cyclohexyldodecanoic acid in lipid bilayer membranes. Chem Phys Lipids. 1983 Dec;34(1):7–24. doi: 10.1016/0009-3084(83)90056-7. [DOI] [PubMed] [Google Scholar]
- Berry A. M., Moreau R. A., Jones A. D. Bacteriohopanetetrol: abundant lipid in frankia cells and in nitrogen-fixing nodule tissue. Plant Physiol. 1991 Jan;95(1):111–115. doi: 10.1104/pp.95.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Hermans M. A., Neuss B., Sahm H. Content and composition of hopanoids in Zymomonas mobilis under various growth conditions. J Bacteriol. 1991 Sep;173(17):5592–5595. doi: 10.1128/jb.173.17.5592-5595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kannenberg E., Poralla K. The influence of hopanoids on growth of Mycoplasma mycoides. Arch Microbiol. 1982 Dec;133(2):100–102. doi: 10.1007/BF00413519. [DOI] [PubMed] [Google Scholar]
- Kelly R., Miller S. M., Lai M. H., Kirsch D. R. Cloning and characterization of the 2,3-oxidosqualene cyclase-coding gene of Candida albicans. Gene. 1990 Mar 15;87(2):177–183. doi: 10.1016/0378-1119(90)90299-7. [DOI] [PubMed] [Google Scholar]
- Kessler C., Höltke H. J., Seibl R., Burg J., Mühlegger K. Non-radioactive labeling and detection of nucleic acids. I. A novel DNA labeling and detection system based on digoxigenin: anti-digoxigenin ELISA principle (digoxigenin system). Biol Chem Hoppe Seyler. 1990 Oct;371(10):917–927. doi: 10.1515/bchm3.1990.371.2.917. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin J. H., Savage D. C. Cloning, nucleotide sequence, and taxonomic implications of the flagellin gene of Roseburia cecicola. J Bacteriol. 1988 Jun;170(6):2612–2617. doi: 10.1128/jb.170.6.2612-2617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs D., Tappe C. H., Gärtner P., Kellner R., Poralla K. Properties of purified squalene-hopene cyclase from Bacillus acidocaldarius. Eur J Biochem. 1990 Nov 26;194(1):75–80. doi: 10.1111/j.1432-1033.1990.tb19429.x. [DOI] [PubMed] [Google Scholar]
- Ourisson G., Rohmer M., Poralla K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu Rev Microbiol. 1987;41:301–333. doi: 10.1146/annurev.mi.41.100187.001505. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Poralla K., Kannenberg E., Blume A. A glycolipid containing hopane isolated from the acidophilic, thermophilic Bacillus acidocaldarius, has a cholesterol-like function in membranes. FEBS Lett. 1980 Apr 21;113(1):107–110. doi: 10.1016/0014-5793(80)80506-0. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Renz M. An optimized freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem. 1983 Jul 1;132(1):14–19. doi: 10.1016/0003-2697(83)90419-0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]