Abstract
A novel multicellular form of Methanosarcina mazei S-6 is described. It was termed lamina, and it formed during the exponential growth phase when packets or single cells were grown in 40 mM trimethylamine and a total concentration of 8.3 to 15.6 mM Ca2+ and/or Mg2+, in cultures that were not shaken. A distinct molecular event represented by the increment in expression and a spatial redistribution of an antigen during lamina formation is documented.
Full text
PDF
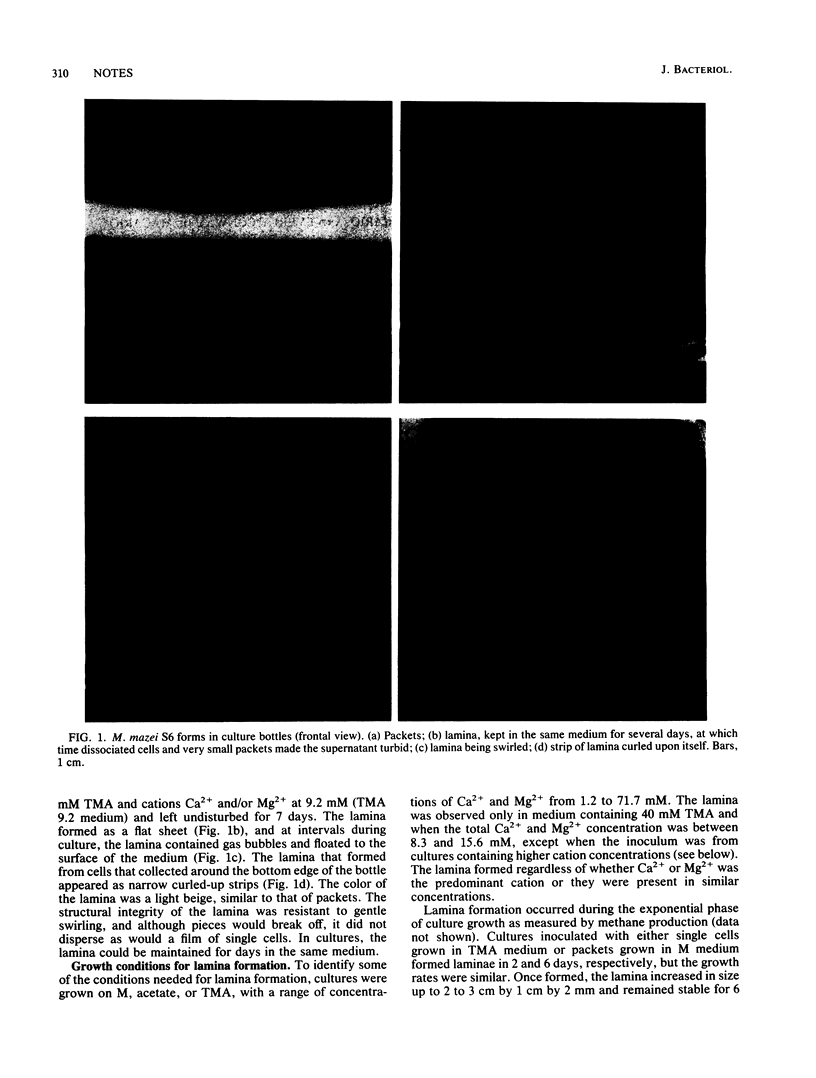
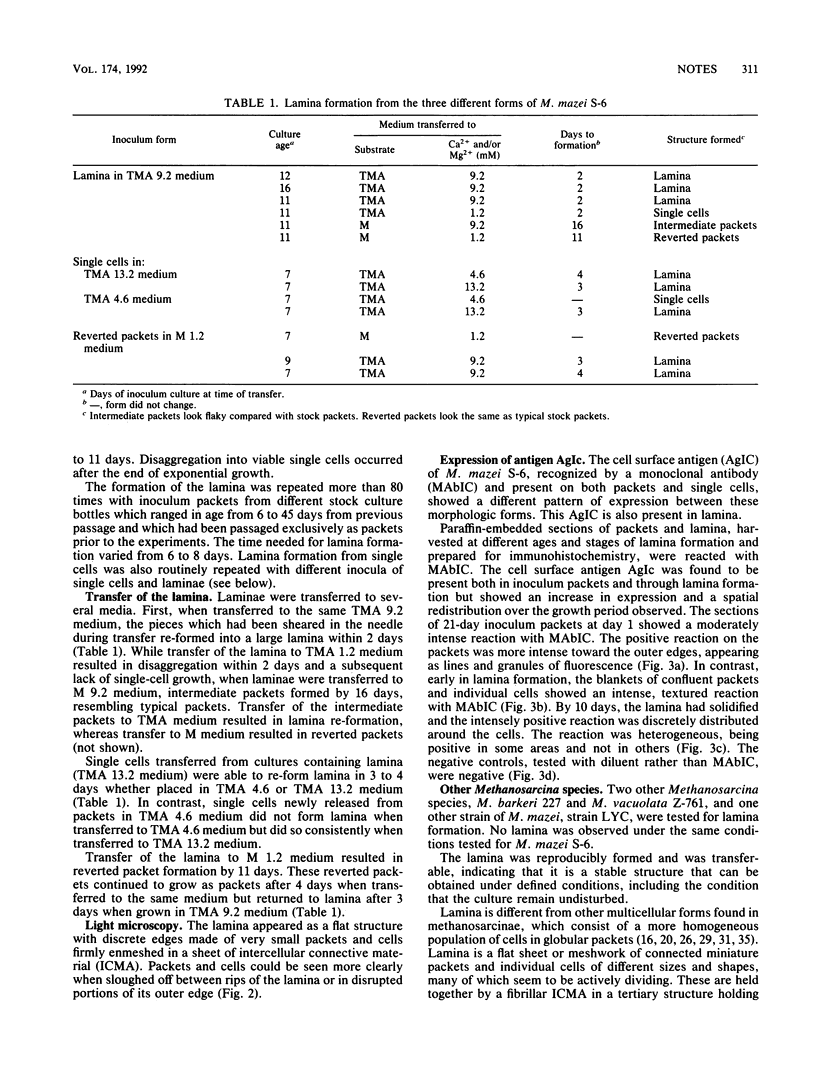



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone D. R., Mah R. A. Effects of Calcium, Magnesium, pH, and Extent of Growth on the Morphology of Methanosarcina mazei S-6. Appl Environ Microbiol. 1987 Jul;53(7):1699–1700. doi: 10.1128/aem.53.7.1699-1700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Mathrani I. M., Mah R. A. H(2)-CO(2) Recirculation and pH Control for Growth of Methanogens in Mass Culture. Appl Environ Microbiol. 1987 May;53(5):946–948. doi: 10.1128/aem.53.5.946-948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Xun L. Effects of pH, Temperature, and Nutrients on Propionate Degradation by a Methanogenic Enrichment Culture. Appl Environ Microbiol. 1987 Jul;53(7):1589–1592. doi: 10.1128/aem.53.7.1589-1592.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codogno P., Doyennette-Moyne M. A., Aubery M., Dieckhoff J., Lietzke R., Mannherz H. G. Polyclonal and monoclonal antibodies against chicken gizzard 5'-nucleotidase inhibit the spreading process of chicken embryonic fibroblasts on laminin substratum. Exp Cell Res. 1988 Feb;174(2):344–354. doi: 10.1016/0014-4827(88)90305-9. [DOI] [PubMed] [Google Scholar]
- Conway de Macario E., Macario A. J., Jovell R. J. Slide immunoenzymatic assay (SIA) in hybridoma technology. Methods Enzymol. 1986;121:509–525. doi: 10.1016/0076-6879(86)21051-4. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Morphoregulatory molecules. Biochemistry. 1988 May 17;27(10):3533–3543. doi: 10.1021/bi00410a001. [DOI] [PubMed] [Google Scholar]
- Gill J. S., Dworkin M. Cell surface antigens during submerged development of Myxococcus xanthus examined with monoclonal antibodies. J Bacteriol. 1986 Nov;168(2):505–511. doi: 10.1128/jb.168.2.505-511.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. E. Spontaneous Disaggregation of Methanosarcina mazei S-6 and Its Use in the Development of Genetic Techniques for Methanosarcina spp. Appl Environ Microbiol. 1987 Oct;53(10):2500–2504. doi: 10.1128/aem.53.10.2500-2504.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Boone D. R., Sleat R., Mah R. A. Methanosarcina mazei LYC, a New Methanogenic Isolate Which Produces a Disaggregating Enzyme. Appl Environ Microbiol. 1985 Mar;49(3):608–613. doi: 10.1128/aem.49.3.608-613.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario A. J., Conway de Macario E., Ney U., Schoberth S. M., Sahm H. Shifts in methanogenic subpopulations measured with antibody probes in a fixed-bed loop anaerobic bioreactor treating sulfite evaporator condensate. Appl Environ Microbiol. 1989 Aug;55(8):1996–2001. doi: 10.1128/aem.55.8.1996-2001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. W., Aldrich H. C., Hurst S. F., Bleiweis A. S. Role of the Cell Surface of Methanosarcina mazei in Cell Aggregation. Appl Environ Microbiol. 1985 Feb;49(2):321–327. doi: 10.1128/aem.49.2.321-327.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. W. Life Cycles in the Methanogenic Archaebacterium Methanosarcina mazei. Appl Environ Microbiol. 1986 Jul;52(1):17–27. doi: 10.1128/aem.52.1.17-27.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Control of morphogenesis in myxobacteria. Crit Rev Microbiol. 1987;14(3):195–227. doi: 10.3109/10408418709104439. [DOI] [PubMed] [Google Scholar]
- Sowers K. R., Gunsalus R. P. Adaptation for growth at various saline concentrations by the archaebacterium Methanosarcina thermophila. J Bacteriol. 1988 Feb;170(2):998–1002. doi: 10.1128/jb.170.2.998-1002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun L. Y., Mah R. A., Boone D. R. Isolation and characterization of disaggregatase from Methanosarcina mazei LYC. Appl Environ Microbiol. 1990 Dec;56(12):3693–3698. doi: 10.1128/aem.56.12.3693-3698.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun L., Boone D. R., Mah R. A. Control of the Life Cycle of Methanosarcina mazei S-6 by Manipulation of Growth Conditions. Appl Environ Microbiol. 1988 Aug;54(8):2064–2068. doi: 10.1128/aem.54.8.2064-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Palm P., Reiter W. D., Gropp F., Pühler G., Klenk H. P. Comparative evaluation of gene expression in archaebacteria. Eur J Biochem. 1988 May 2;173(3):473–482. doi: 10.1111/j.1432-1033.1988.tb14023.x. [DOI] [PubMed] [Google Scholar]





