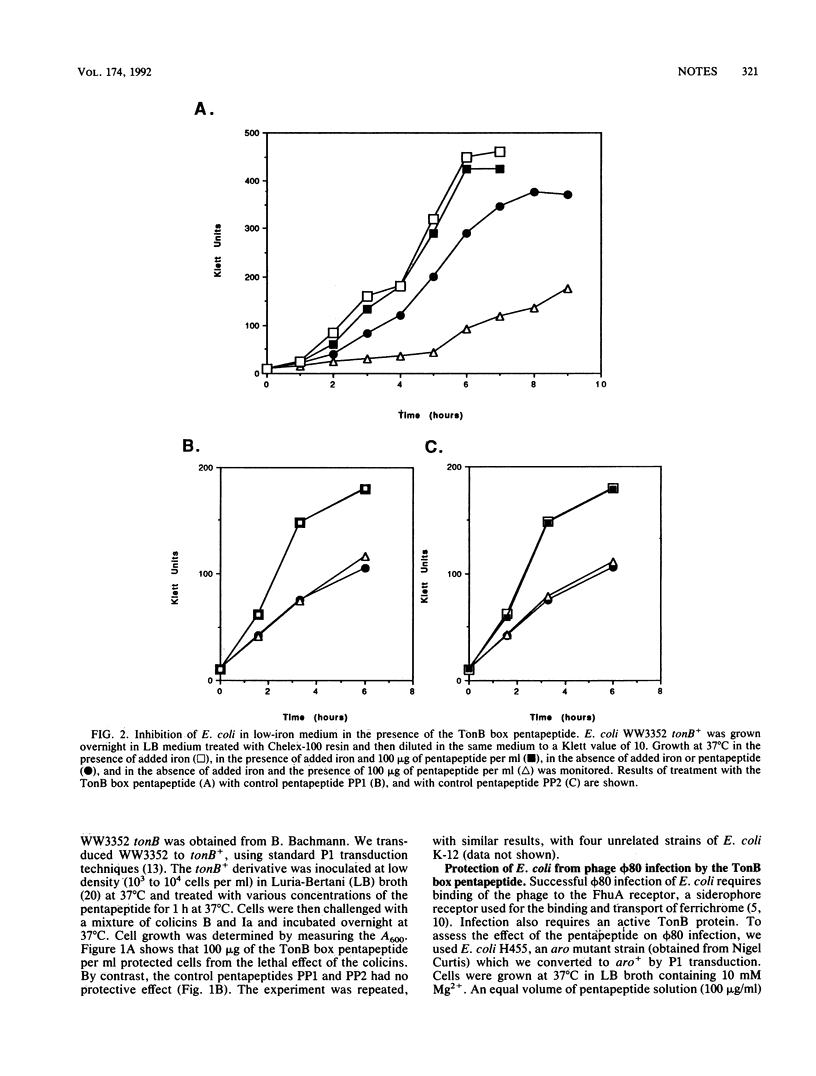
Abstract
The TonB box, a conserved pentapeptide sequence found in TonB-dependent colicins and receptors, is thought to interact physically with the TonB protein to facilitate TonB-dependent processes. Strains of Escherichia coli were treated in vivo with the synthetic TonB box pentapeptide Glu-Thr-Val-Ile-Val. The pentapeptide inhibited several TonB-dependent processes, including cell growth in low-iron medium, phi 80 infection, and killing by colicins B and Ia. Two unrelated control pentapeptides had no effect on TonB-dependent processes.
Full text
PDF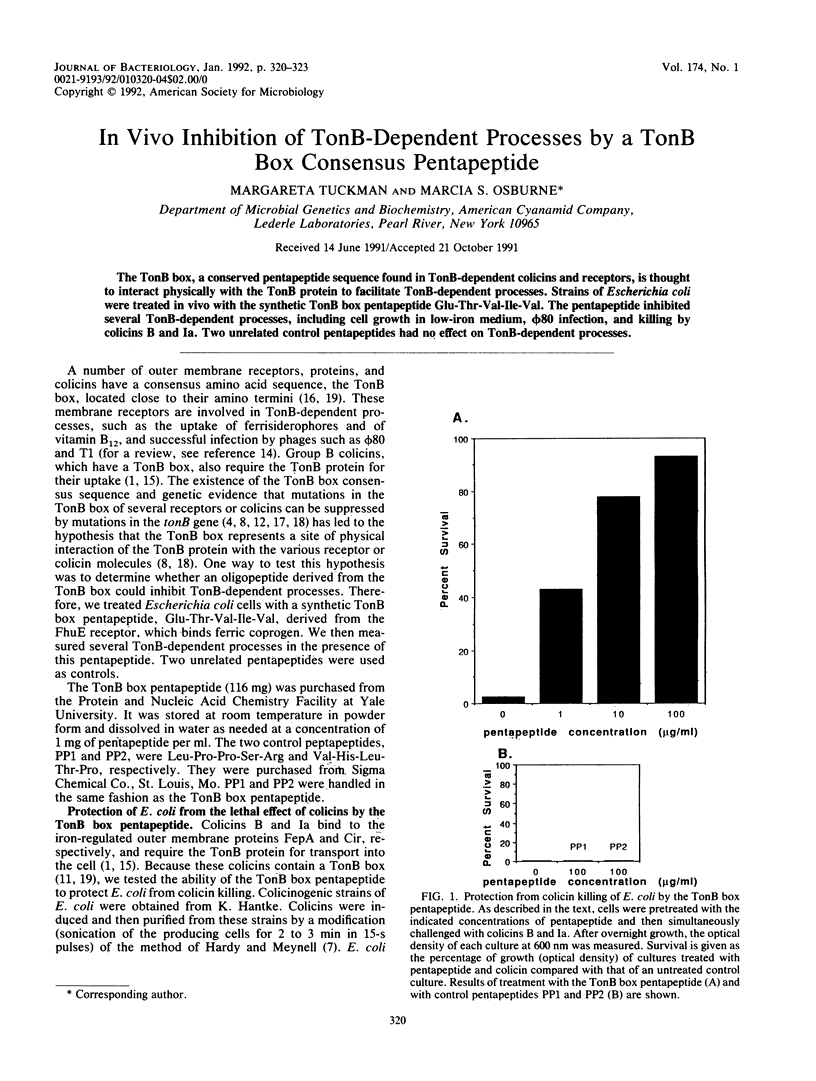


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Frenz J., Hantke K., Schaller K. Penetration of colicin M into cells of Escherichia coli. J Bacteriol. 1980 Apr;142(1):162–168. doi: 10.1128/jb.142.1.162-168.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer S., Tolley M., Trayer I. P., Barr G. C., Dorman C. J., Hannavy K., Higgins C. F., Evans J. S., Levine B. A., Wormald M. R. Structure and function of X-Pro dipeptide repeats in the TonB proteins of Salmonella typhimurium and Escherichia coli. J Mol Biol. 1990 Dec 20;216(4):883–895. doi: 10.1016/S0022-2836(99)80008-4. [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir A., Bell P. E., Lundrigan M. D., Bradbeer C., Kadner R. J. Point mutations in a conserved region (TonB box) of Escherichia coli outer membrane protein BtuB affect vitamin B12 transport. J Bacteriol. 1989 Dec;171(12):6526–6533. doi: 10.1128/jb.171.12.6526-6533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Hardy K. G., Meynell G. G. "Induction" of colicin factor E2-P9 by mitomycin C. J Bacteriol. 1972 Nov;112(2):1007–1009. doi: 10.1128/jb.112.2.1007-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. J., Kadner R. J., Günther K. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene. 1988 Apr 15;64(1):147–153. doi: 10.1016/0378-1119(88)90488-x. [DOI] [PubMed] [Google Scholar]
- Hogarth B. G., Higgins C. F. Genetic organization of the oligopeptide permease (opp) locus of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Mar;153(3):1548–1551. doi: 10.1128/jb.153.3.1548-1551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Wayne R., Neilands J. B. In vitro competition between ferrichrome and phage for the outer membrane T5 receptor complex of Escherichia coli. Biochem Biophys Res Commun. 1975 May 19;64(2):687–693. doi: 10.1016/0006-291x(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Mankovich J. A., Hsu C. H., Konisky J. DNA and amino acid sequence analysis of structural and immunity genes of colicins Ia and Ib. J Bacteriol. 1986 Oct;168(1):228–236. doi: 10.1128/jb.168.1.228-236.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende J., Braun V. Import-defective colicin B derivatives mutated in the TonB box. Mol Microbiol. 1990 Sep;4(9):1523–1533. doi: 10.1111/j.1365-2958.1990.tb02063.x. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P. The ins and outs of colicins. Part I: Production, and translocation across membranes. Microbiol Sci. 1984 Oct;1(7):168–175. [PubMed] [Google Scholar]
- Roos U., Harkness R. E., Braun V. Assembly of colicin genes from a few DNA fragments. Nucleotide sequence of colicin D. Mol Microbiol. 1989 Jul;3(7):891–902. doi: 10.1111/j.1365-2958.1989.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Sequence of the fhuE outer-membrane receptor gene of Escherichia coli K12 and properties of mutants. Mol Microbiol. 1990 Mar;4(3):427–437. doi: 10.1111/j.1365-2958.1990.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Schramm E., Mende J., Braun V., Kamp R. M. Nucleotide sequence of the colicin B activity gene cba: consensus pentapeptide among TonB-dependent colicins and receptors. J Bacteriol. 1987 Jul;169(7):3350–3357. doi: 10.1128/jb.169.7.3350-3357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffler H., Braun V. Transport across the outer membrane of Escherichia coli K12 via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol Gen Genet. 1989 Jun;217(2-3):378–383. doi: 10.1007/BF02464907. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Cami B., Brevet J., Cote R. Isolation and characterization of rifampin-resistant and streptolydigin-resistant mutants of Bacillus subtilis with altered sporulation properties. J Bacteriol. 1974 Oct;120(1):253–265. doi: 10.1128/jb.120.1.253-265.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


