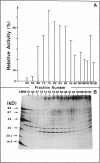
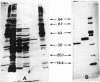
Abstract
An LD-carboxypeptidase releasing the terminal D-Ala from UDP-MurNAc-L-Ala-D-Glu-m-A2pm-D-Ala (UDP-MurNAc-tetrapeptide) was purified from Escherichia coli to biochemical homogeneity and characterized biochemically. Final purification was achieved by nocardicin A-Sepharose affinity chromatography. An apparent molecular weight of 32,000 was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the enzyme, which seems to be a monomeric protein as indicated by gel filtration. The optimum pH of the enzyme was 8.4, and the pI was 5.5. The Km for UDP-MurNAc-tetrapeptide was 1.5 x 10(-4) M, and the Vmax was 0.4 nmol/min. Nocardicin A inhibited the enzyme competitively, with a Ki of 5 x 10(-5) M. Benzylpenicillin, cephalosporin C, thienamycin, and D-alanyl-D-alanine did not affect the enzyme activity. Possible functions of the enzyme for growth and division of the murein sacculus are discussed.
Full text
PDF

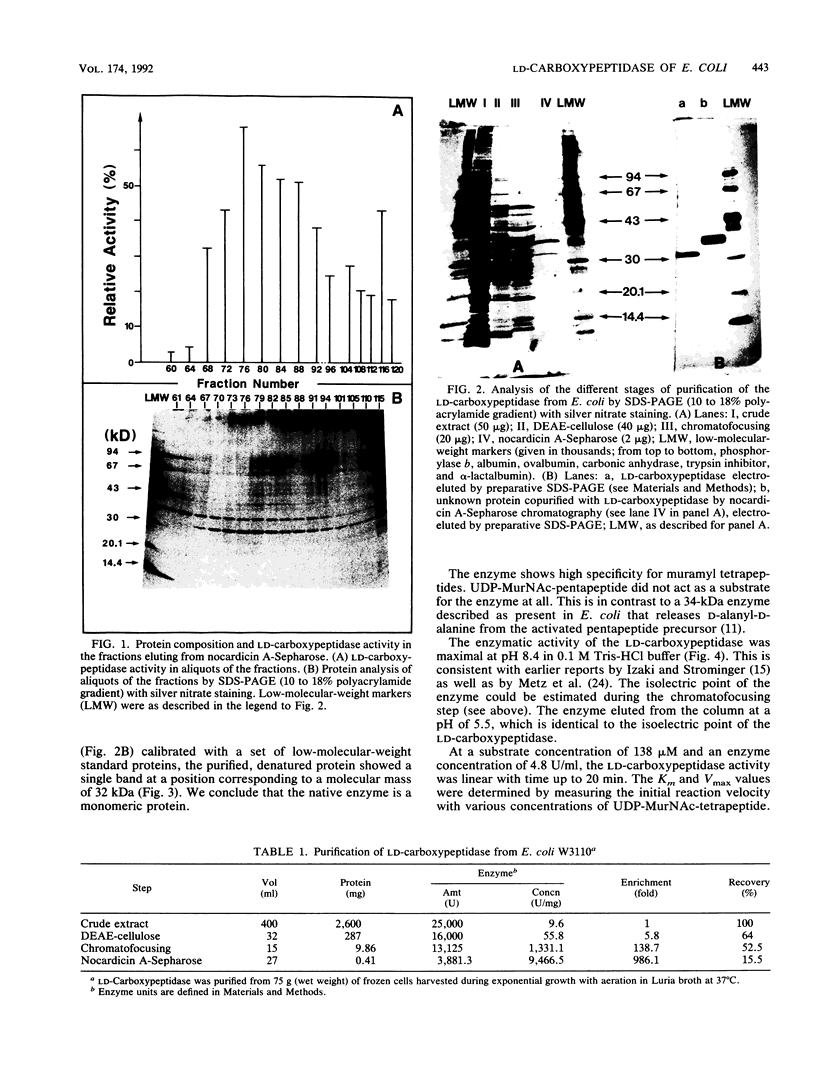



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck B. D., Park J. T. Activity of three murein hydrolases during the cell division cycle of Escherichia coli K-12 as measured in toluene-treated cells. J Bacteriol. 1976 Jun;126(3):1250–1260. doi: 10.1128/jb.126.3.1250-1260.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B. D., Park J. T. Basis for the observed fluctuation of carboxypeptidase II activity during the cell cycle in BUG 6, a temperature-sensitive division mutant of Escherichia coli. J Bacteriol. 1977 Jun;130(3):1292–1302. doi: 10.1128/jb.130.3.1292-1302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Takasuga A., Edwards D. H., Dewar S. J., Spratt B. G., Adachi H., Ohta T., Matsuzawa H., Donachie W. D. The balance between different peptidoglycan precursors determines whether Escherichia coli cells will elongate or divide. J Bacteriol. 1990 Dec;172(12):6697–6703. doi: 10.1128/jb.172.12.6697-6703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. Attachment of lipoprotein to murein (peptidoglycan) of Escherichia coli in the presence and absence of penicillin FL 1060. J Bacteriol. 1975 Sep;123(3):888–897. doi: 10.1128/jb.123.3.888-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Wolff H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem. 1970 Jun;14(2):387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Fung J., MacAlister T. J., Rothfield L. I. Role of murein lipoprotein in morphogenesis of the bacterial division septum: phenotypic similarity of lkyD and lpo mutants. J Bacteriol. 1978 Mar;133(3):1467–1471. doi: 10.1128/jb.133.3.1467-1471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Frère J. M., Leyh-Bouille M., Coyette J., Dusart J., Nguyen-Distèche M. Use of model enzymes in the determination of the mode of action of penicillins and delta 3-cephalosporins. Annu Rev Biochem. 1979;48:73–101. doi: 10.1146/annurev.bi.48.070179.000445. [DOI] [PubMed] [Google Scholar]
- Glauner B., Höltje J. V. Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem. 1990 Nov 5;265(31):18988–18996. [PubMed] [Google Scholar]
- Gondré B., Flouret B., van Heijenoort J. Release of D-alanyl-D-alanine from the precursor of the cell wall peptidoglycan by a peptidase of Escherichia coli K 12. Biochimie. 1973;55(6):685–691. doi: 10.1016/s0300-9084(73)80022-7. [DOI] [PubMed] [Google Scholar]
- Hammes W. P., Seidel H. The LD-carboxypeptidase activity in Gaffkya homari. The target of the action of certain beta-lactam antibiotics on the formation of wall-bound peptidoglycan. Eur J Biochem. 1978 Nov 15;91(2):509–515. doi: 10.1111/j.1432-1033.1978.tb12704.x. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Tuomanen E. I. The murein hydrolases of Escherichia coli: properties, functions and impact on the course of infections in vivo. J Gen Microbiol. 1991 Mar;137(3):441–454. doi: 10.1099/00221287-137-3-441. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XIV. Purification and properties of two D-alanine carboxypeptidases from Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3193–3201. [PubMed] [Google Scholar]
- Kelly J. A., Moews P. C., Knox J. R., Frère J. M., Ghuysen J. M. Penicillin target enzyme and the antibiotic binding site. Science. 1982 Oct 29;218(4571):479–481. doi: 10.1126/science.7123246. [DOI] [PubMed] [Google Scholar]
- Kohlrausch U., Höltje J. V. One-step purification procedure for UDP-N-acetylmuramyl-peptide murein precursors from Bacillus cereus. FEMS Microbiol Lett. 1991 Mar 1;62(2-3):253–257. doi: 10.1016/0378-1097(91)90166-8. [DOI] [PubMed] [Google Scholar]
- Korat B., Mottl H., Keck W. Penicillin-binding protein 4 of Escherichia coli: molecular cloning of the dacB gene, controlled overexpression, and alterations in murein composition. Mol Microbiol. 1991 Mar;5(3):675–684. doi: 10.1111/j.1365-2958.1991.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Markiewicz Z., Broome-Smith J. K., Schwarz U., Spratt B. G. Spherical E. coli due to elevated levels of D-alanine carboxypeptidase. Nature. 1982 Jun 24;297(5868):702–704. doi: 10.1038/297702a0. [DOI] [PubMed] [Google Scholar]
- Metz R., Henning S., Hammes W. P. LD-carboxypeptidase activity in Escherichia coli. I. The LD-carboxypeptidase activity in ether treated cells. Arch Microbiol. 1986 Mar;144(2):175–180. doi: 10.1007/BF00414731. [DOI] [PubMed] [Google Scholar]
- Metz R., Henning S., Hammes W. P. LD-carboxypeptidase activity in Escherichia coli. II. Isolation, purification and characterization of the enzyme from E. coli K 12. Arch Microbiol. 1986 Mar;144(2):181–186. doi: 10.1007/BF00414732. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Regulation of murein biosynthesis and septum formation in filamentous cells of Escherichia coli PAT 84. J Bacteriol. 1977 Mar;129(3):1593–1600. doi: 10.1128/jb.129.3.1593-1600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Strominger J. L. Identification of the major penicillin-binding proteins of Escherichia coli as D-alanine carboxypeptidase IA. J Bacteriol. 1976 Jul;127(1):660–663. doi: 10.1128/jb.127.1.660-663.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink J., Gilden N., Witholt B. Attachment of lipoprotein to the murein of Escherichia coli. Eur J Biochem. 1982 Mar 1;122(3):587–590. doi: 10.1111/j.1432-1033.1982.tb06479.x. [DOI] [PubMed] [Google Scholar]
- Wensink J., Witholt B. Conversion of free lipoprotein to the murein-bound form. Eur J Biochem. 1981 Jun;117(1):207–212. doi: 10.1111/j.1432-1033.1981.tb06323.x. [DOI] [PubMed] [Google Scholar]