Abstract
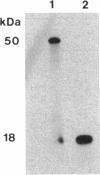
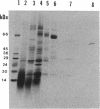
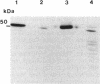
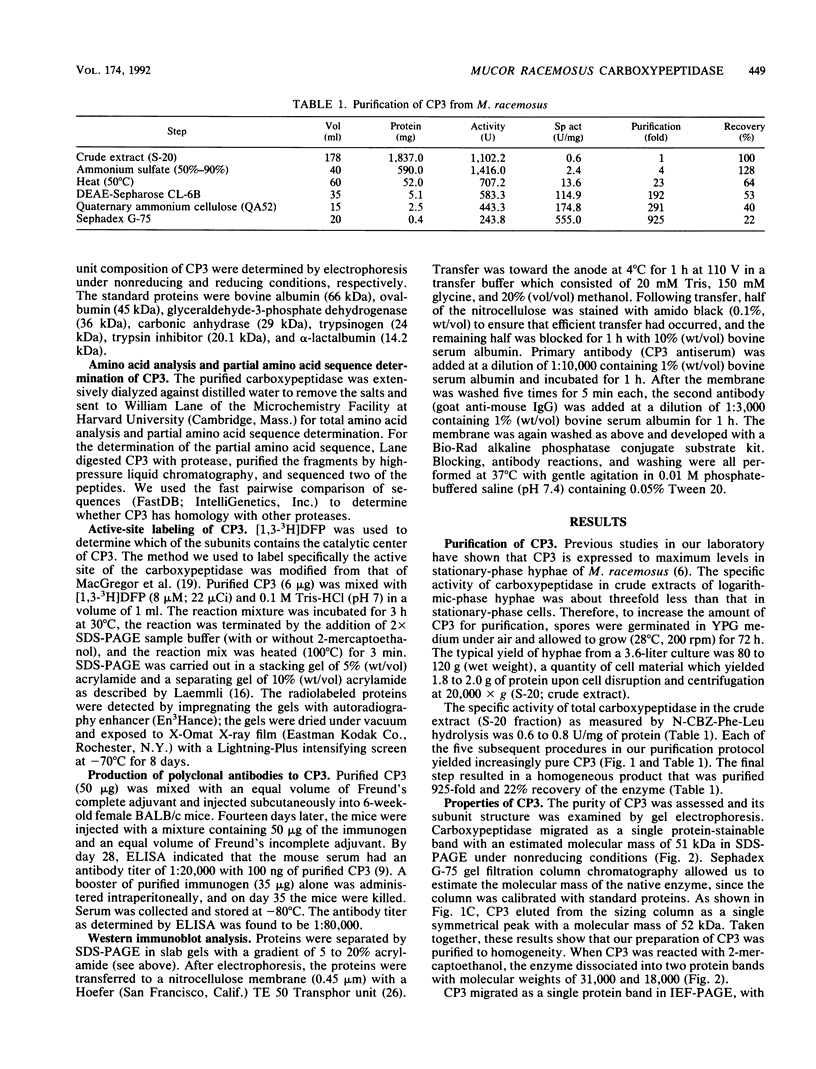
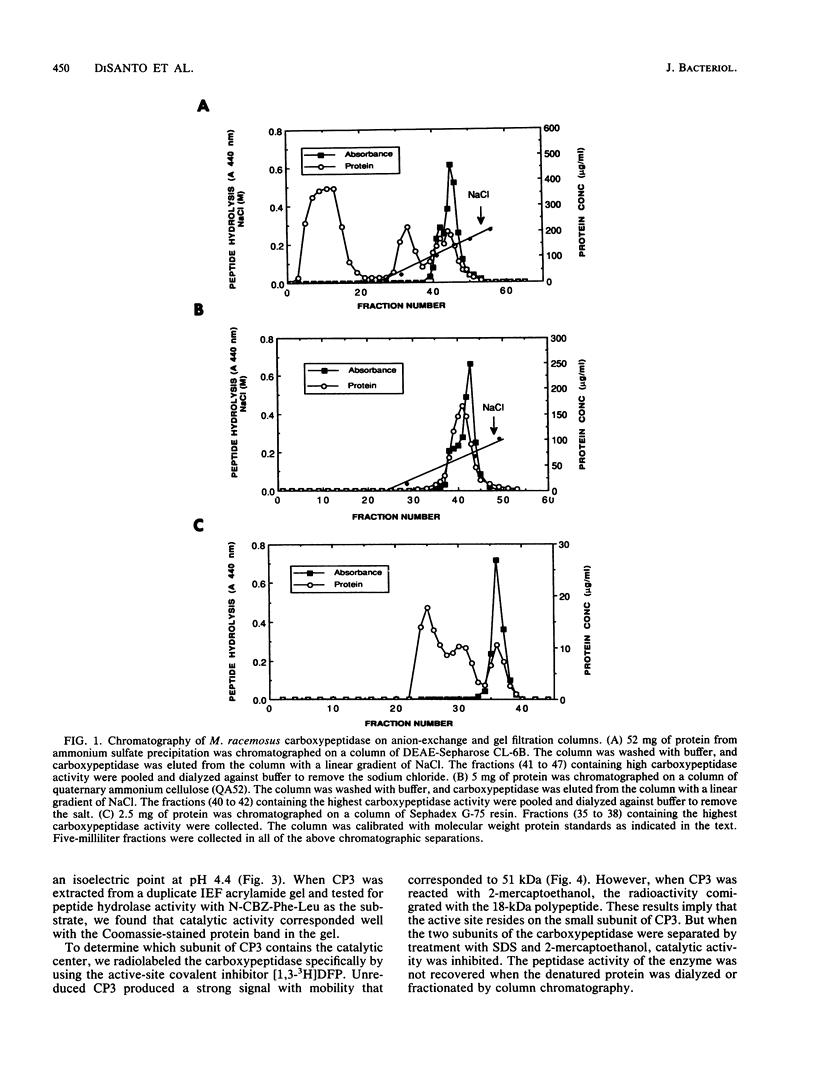
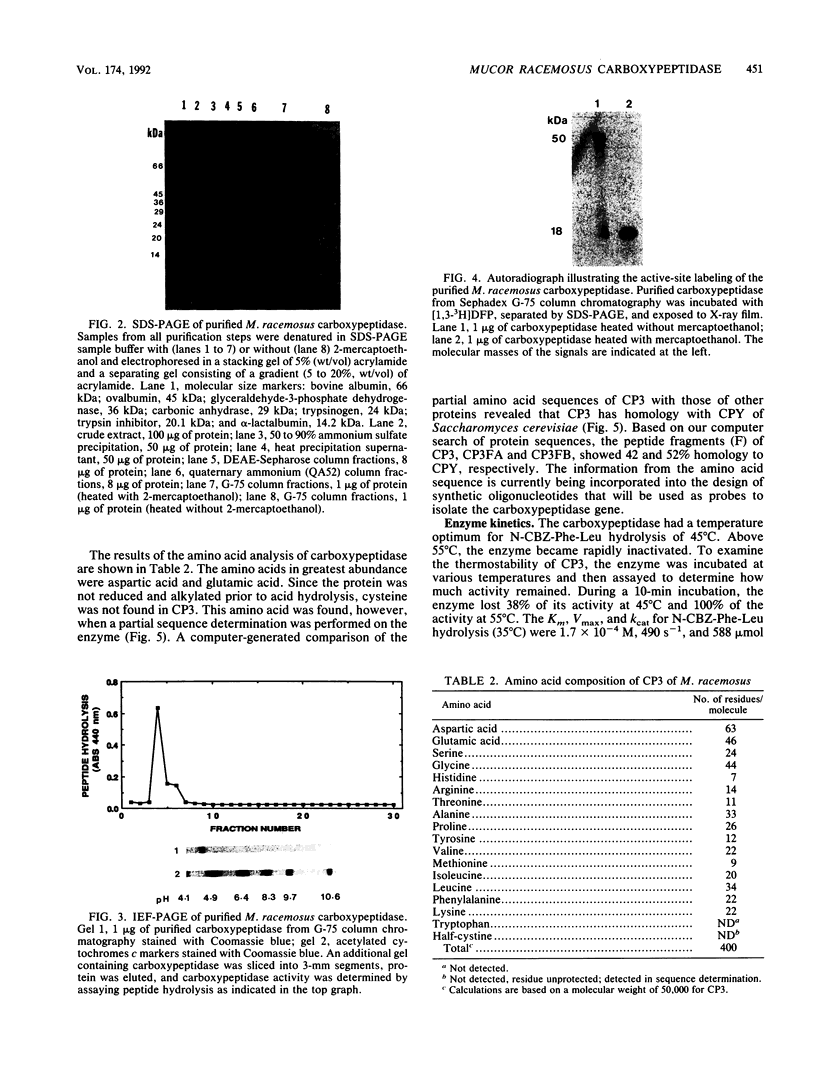
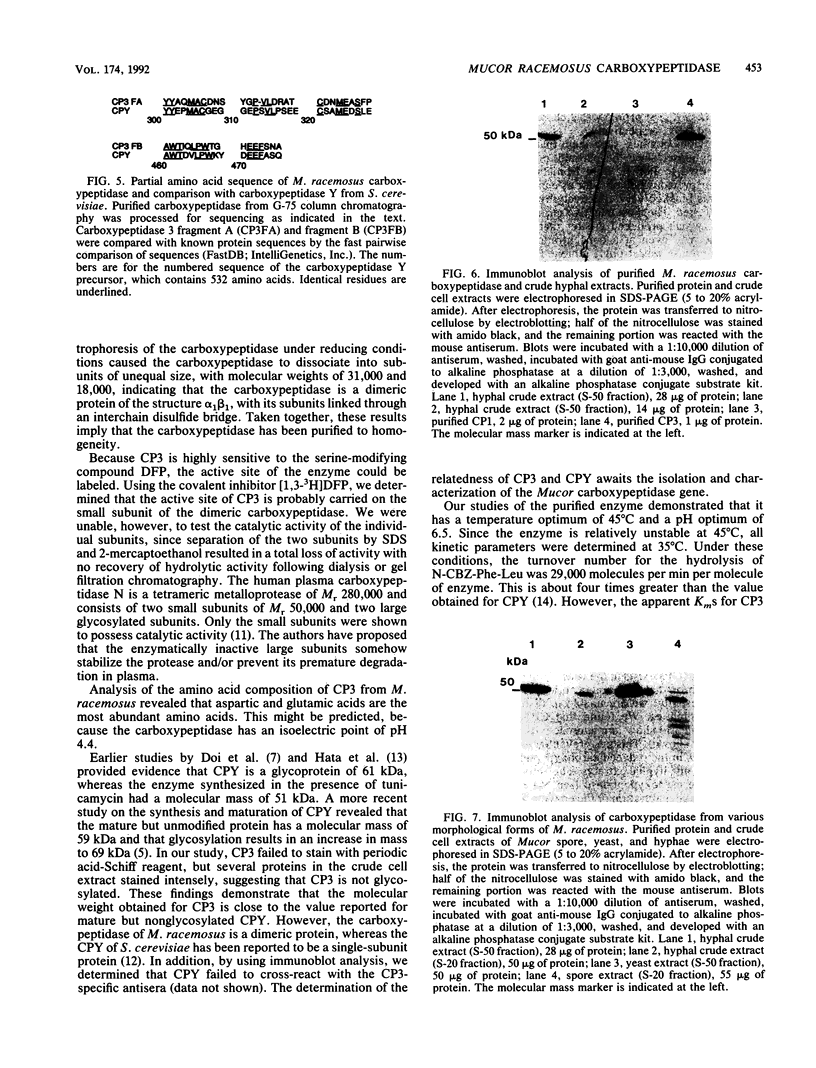
A developmentally regulated carboxypeptidase was purified from hyphae of the dimorphic fungus Mucor racemosus. The enzyme, designated carboxypeptidase 3 (CP3), has been purified greater than 900-fold to homogeneity and characterized. The carboxypeptidase migrated as a single electrophoretic band in isoelectric focusing polyacrylamide gel electrophoresis (PAGE), with an isoelectric point of pH 4.4. The apparent molecular mass of the native enzyme was estimated by gel filtration to be 52 kDa. Sodium dodecyl sulfate (SDS)-PAGE under nonreducing conditions revealed the presence of a single polypeptide of 51 kDa. SDS-PAGE of CP3 reacted with 2-mercaptoethanol revealed the presence of two polypeptides of 31 and 18 kDa, indicating a dimer structure (alpha 1 beta 1) of the enzyme with disulfide-linked subunits. By using [1,3-3H]diisopropylfluorophosphate as an active-site labeling reagent, it was determined that the catalytic site resides on the small subunit of the carboxypeptidase. With N-carboben zoxy-L-phenylalanyl-L-leucine (N-CBZ-Phe-Leu) as the substrate, the Km, kcat, and Vmax values were 1.7 x 10(-4) M, 490 s-1, and 588 mumol of Leu released per min per mg of protein, respectively. CP3 was determined to be a serine protease, since its catalytic activity was blocked by the serine protease inhibitors diisopropylfluorophosphate, phenylmethylsulfonyl fluoride, and 3,4-dichloroi Socoumarin (DCI). The enzyme was strongly inhibited by the mercurial compound p-chloromercuribenzoate. The carboxypeptidase readily hydrolyzed peptides with aliphatic or aromatic side chains, whereas most of the peptides which contained glycine in the penultimate position did not serve as substrates for the enzyme. Although CP3 activity was undetectable in Mucor yeast cells, antisera revealed the presence of the enzyme in the yeast form of the fungus. The partial amino acid sequence of the carboxypeptidase was determined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Wolf D. H. Proteinases, proteolysis and biological control in the yeast Saccharomyces cerevisiae. Yeast. 1985 Dec;1(2):139–157. doi: 10.1002/yea.320010203. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Hayashi R. Properties of the single sulfhydryl group of carboxypeptidase Y. Effects of alkyl and aromatic mercurials on activities toward various synthetic substrates. J Biol Chem. 1979 Sep 10;254(17):8473–8479. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Distel B., Al E. J., Tabak H. F., Jones E. W. Synthesis and maturation of the yeast vacuolar enzymes carboxypeptidase Y and aminopeptidase I. Biochim Biophys Acta. 1983 Oct 13;741(1):128–135. doi: 10.1016/0167-4781(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Emter O., Wolf D. H. Vacuoles are not the sole compartments of proteolytic enzymes in yeast. FEBS Lett. 1984 Jan 30;166(2):321–325. doi: 10.1016/0014-5793(84)80104-0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Fujita M., Parsons D. S., Wojnarowska F. Oligopeptidases of brush border membranes of rat small intestinal mucosal cells. J Physiol. 1972 Dec;227(2):377–394. doi: 10.1113/jphysiol.1972.sp010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard W., Schube M., Eulitz M. cDNA cloning and complete primary structure of the small, active subunit of human carboxypeptidase N (kininase 1). Eur J Biochem. 1989 Jan 2;178(3):603–607. doi: 10.1111/j.1432-1033.1989.tb14488.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Carbohydrate moiety of carboxypeptidase Y and perturbation of its biosynthesis. Eur J Biochem. 1978 Nov 15;91(2):567–575. doi: 10.1111/j.1432-1033.1978.tb12710.x. [DOI] [PubMed] [Google Scholar]
- Hayashi R. Carboxypeptidase Y. Methods Enzymol. 1976;45:568–587. doi: 10.1016/s0076-6879(76)45051-6. [DOI] [PubMed] [Google Scholar]
- Jones E. W. The synthesis and function of proteases in Saccharomyces: genetic approaches. Annu Rev Genet. 1984;18:233–270. doi: 10.1146/annurev.ge.18.120184.001313. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenney J. F. Three yeast proteins that specifically inhibit yeast proteases A, B, and C. J Bacteriol. 1975 Jun;122(3):1265–1273. doi: 10.1128/jb.122.3.1265-1273.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Bishop C. W., Blech J. E. Localization of proteolytic activity in the outer membrane of Escherichia coli. J Bacteriol. 1979 Jan;137(1):574–583. doi: 10.1128/jb.137.1.574-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern H., Hoffmann M., Holzer H. Isolation and characterization of the carboxypeptidase Y inhibitor from yeast. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4874–4878. doi: 10.1073/pnas.71.12.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki T., Matsuda Y., Holzer H. Urification and characterization of macromolecular inhibitors of proteinase A from yeast. Eur J Biochem. 1974 Sep 1;47(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03697.x. [DOI] [PubMed] [Google Scholar]
- Spormann D. O., Heim J., Wolf D. H. Carboxypeptidase yscS: gene structure and function of the vacuolar enzyme. Eur J Biochem. 1991 Apr 23;197(2):399–405. doi: 10.1111/j.1432-1033.1991.tb15924.x. [DOI] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S., Borgia P. T., Paznokas J. L. Biochemistry of dimorphism in the fungus Mucor. Adv Microb Physiol. 1978;18:67–104. doi: 10.1016/s0065-2911(08)60415-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. H. Control of metabolism in yeast and other lower eukaryotes through action of proteinases. Adv Microb Physiol. 1980;21:267–338. doi: 10.1016/s0065-2911(08)60358-6. [DOI] [PubMed] [Google Scholar]