Abstract
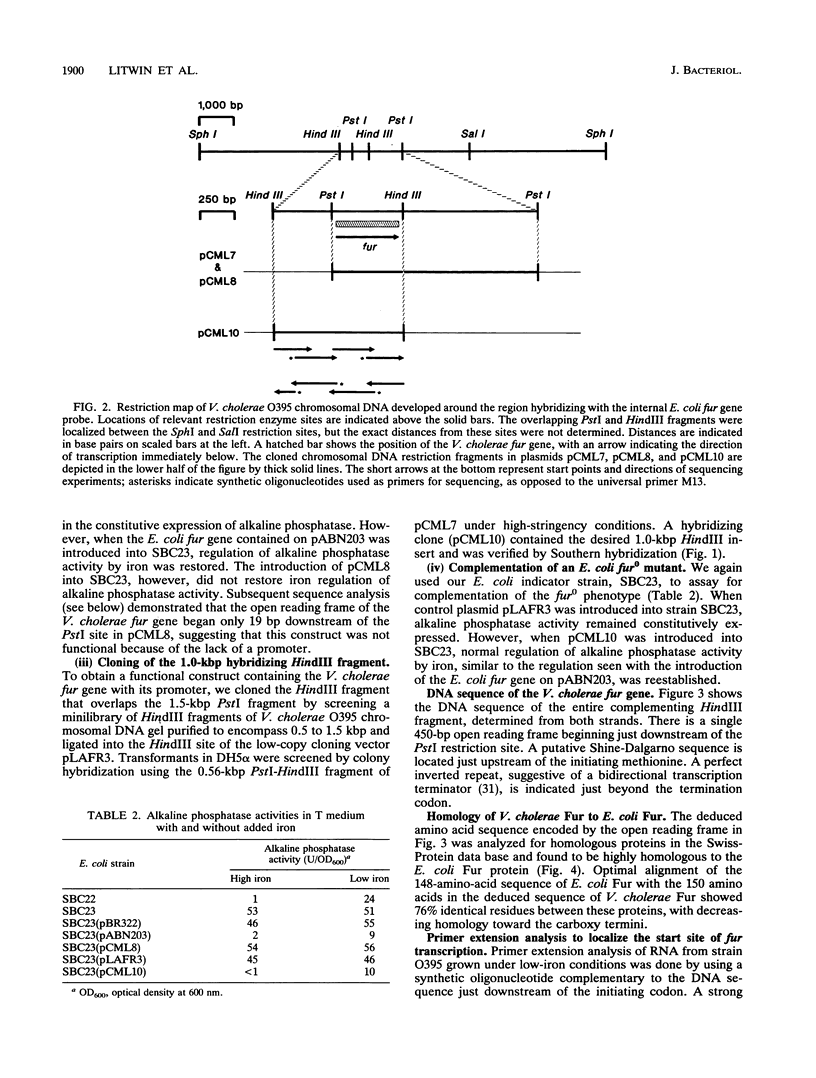
Many genes involved in the transport of iron by bacteria as well as in pathogenesis are regulated by the environmental concentration of iron, with increased expression under low-iron conditions. In Escherichia coli, transcriptional regulation by iron depends on the fur gene. A virulence gene in Vibrio cholerae (irgA) is also transcriptionally regulated by iron, and the promoter of irgA contains a dyad repeat homologous to Fur binding sites in E. coli. Southern hybridization of V. cholerae chromosomal DNA, using an internal fragment of E. coli fur as a probe, showed a single hybridizing sequence under conditions of low stringency. We derived a restriction map in the vicinity of this hybridizing sequence; overlapping PstI and HindIII fragments were identified at the center of this map. The cloned PstI fragment failed to complement an E. coli fur mutant; sequence analysis revealed an open reading frame that began just downstream of the PstI site, suggesting that the clone was not functional because it lacked its promoter. The overlapping HindIII fragment contained the intact V. cholerae fur gene with its promoter and complemented an E. coli fur mutant. DNA sequencing of the HindIII fragment demonstrated a single open reading frame of 150 amino acids that was 76% homologous to E. coli Fur. Primer extension analysis localized two promoters for the V. cholerae fur gene; no significant homology to an E. coli Fur binding site was identified for either promoter. Northern blot analysis showed that the two fur transcripts were not strongly regulated by iron. These studies identify a gene in V. cholerae homologous in both function and sequence to the fur gene of E. coli, and we have designated this gene the fur gene of V. cholerae.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Actis L. A., Potter S. A., Crosa J. H. Iron-regulated outer membrane protein OM2 of Vibrio anguillarum is encoded by virulence plasmid pJM1. J Bacteriol. 1985 Feb;161(2):736–742. doi: 10.1128/jb.161.2.736-742.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorn M. J., Iglewski B. H., Ives S. K., Sadoff J. C., Vasil M. L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978 Mar;19(3):785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J., Oza M. N., Murphy J. R. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J. The significance of iron in infection. Rev Infect Dis. 1981 Nov-Dec;3(6):1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Confirmation of the Fur operator site by insertion of a synthetic oligonucleotide into an operon fusion plasmid. J Bacteriol. 1988 Feb;170(2):1015–1017. doi: 10.1128/jb.170.2.1015-1017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel E., Mazigh D., Mollaret H. H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987 Jan;55(1):277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grandis S., Ginsberg J., Toone M., Climie S., Friesen J., Brunton J. Nucleotide sequence and promoter mapping of the Escherichia coli Shiga-like toxin operon of bacteriophage H-19B. J Bacteriol. 1987 Sep;169(9):4313–4319. doi: 10.1128/jb.169.9.4313-4319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo V., Herrero M., Giovannini F., Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988 May 2;173(3):537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- Ernst J. F., Bennett R. L., Rothfield L. I. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978 Sep;135(3):928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., Boyko S. A., Calderwood S. B. Positive transcriptional regulation of an iron-regulated virulence gene in Vibrio cholerae. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1125–1129. doi: 10.1073/pnas.88.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., Boyko S. A., Calderwood S. B. Transcriptional regulation by iron of a Vibrio cholerae virulence gene and homology of the gene to the Escherichia coli fur system. J Bacteriol. 1990 Dec;172(12):6863–6870. doi: 10.1128/jb.172.12.6863-6870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. B., DiRita V. J., Calderwood S. B. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect Immun. 1990 Jan;58(1):55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Inouye H., Oliver D., Beckwith J. Mutations that alter the signal sequence of alkaline phosphatase in Escherichia coli. J Bacteriol. 1983 Apr;154(1):366–374. doi: 10.1128/jb.154.1.366-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Landfear S. M., Wirth D. F. Cloning and characterization of a Leishmania gene encoding a RNA spliced leader sequence. Nucleic Acids Res. 1986 Sep 25;14(18):7341–7360. doi: 10.1093/nar/14.18.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Braun V. Iron regulation of Serratia marcescens hemolysin gene expression. Infect Immun. 1988 Nov;56(11):2967–2971. doi: 10.1128/iai.56.11.2967-2971.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffer S., Hantke K., Braun V. Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet. 1985;200(1):110–113. doi: 10.1007/BF00383321. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staggs T. M., Perry R. D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991 Jan;173(2):417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzman A., Kapoor S., Graham A. F., Meighen E. A. A new Vibrio fischeri lux gene precedes a bidirectional termination site for the lux operon. J Bacteriol. 1990 Dec;172(12):6797–6802. doi: 10.1128/jb.172.12.6797-6802.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S., Neilands J. B., Bittner M. L., Hemming B. C., Haymore B. L., Seetharam R. Expression, isolation and properties of Fur (ferric uptake regulation) protein of Escherichia coli K 12. Biol Met. 1988;1(1):62–68. doi: 10.1007/BF01128019. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]