Abstract
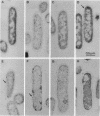
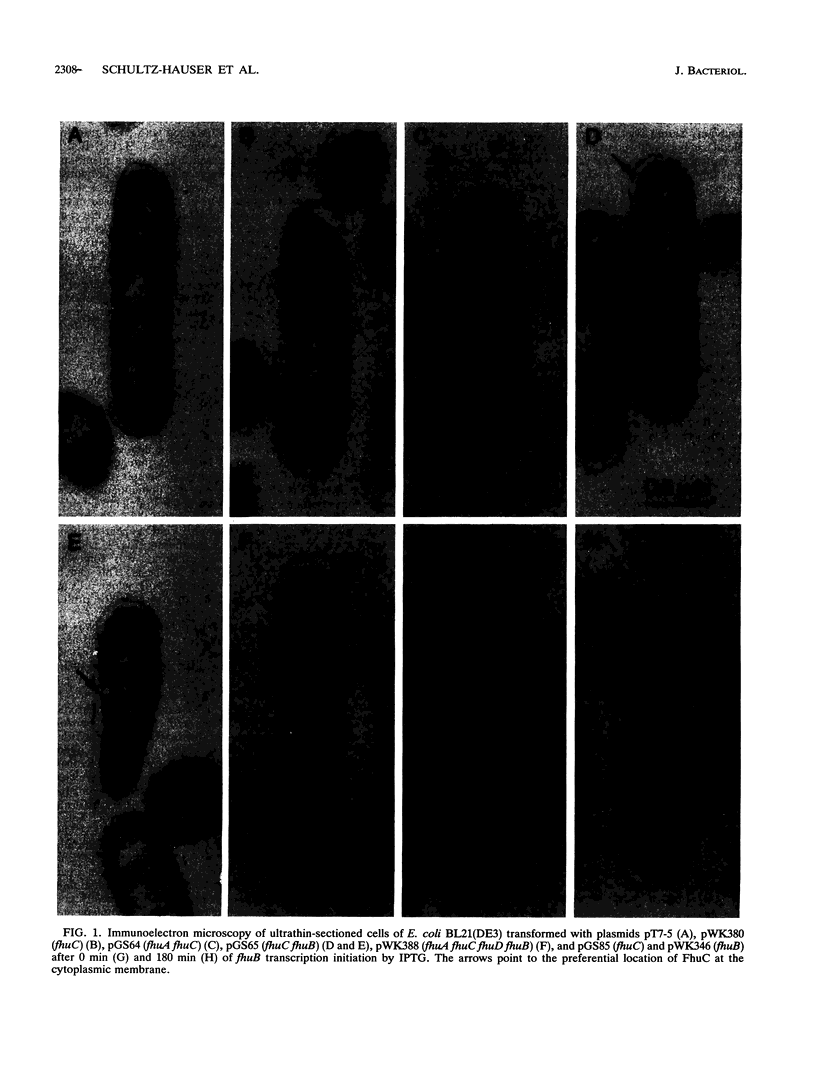
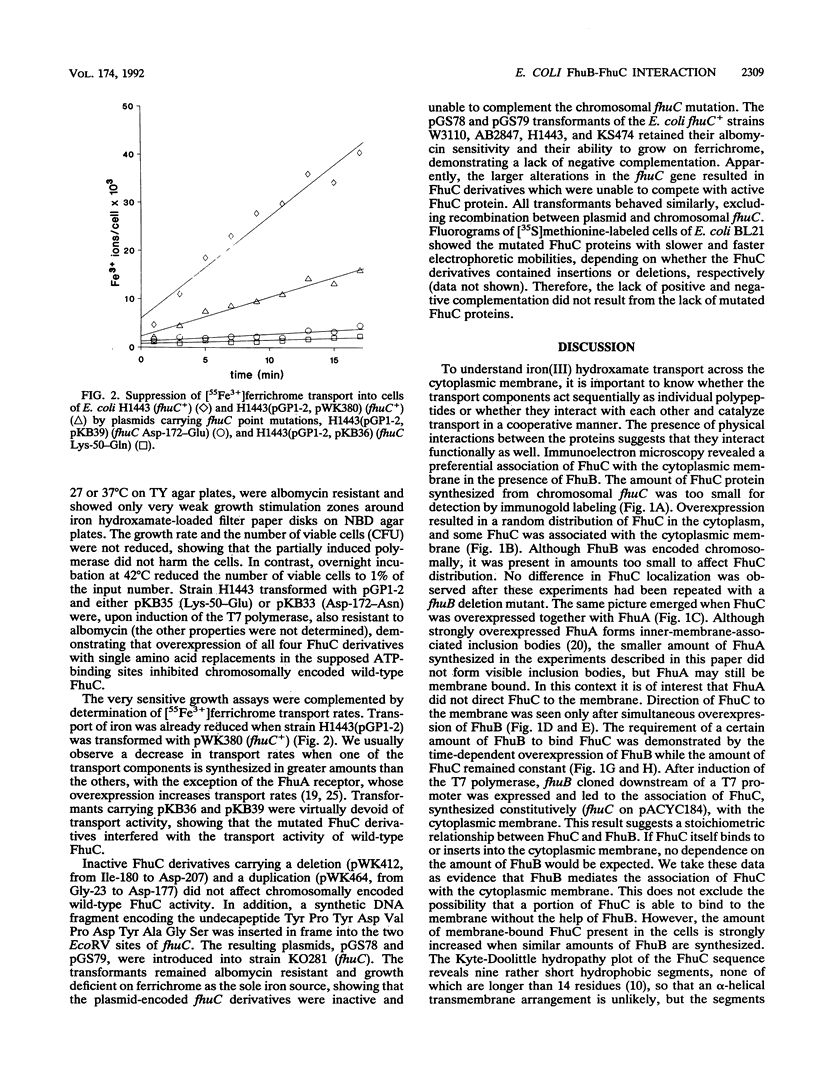
Iron(III) hydroxamate transport across the cytoplasmic membrane is catalyzed by the very hydrophobic FhuB protein and the membrane-associated FhuC protein, which contains typical ATP-binding domains. Interaction between the two proteins was demonstrated by immunoelectron microscopy with anti-FhuC antibodies, which showed FhuB-mediated association of FhuC with the cytoplasmic membrane. In addition, inactive FhuC derivatives carrying single amino acid replacements in the ATP-binding domains suppressed wild-type FhuC transport activity, which arose either from displacement of active FhuC from FhuB by the mutated FhuC derivatives or from the formation of mixed inactive FhuC multimers between wild-type and mutated FhuC proteins. Inactive FhuC derivatives containing internal deletions and insertions showed no phenotypic suppression, indicating conformational alterations that rendered the FhuC derivatives unable to displace wild-type FhuC. It is concluded that the physical interaction between FhuC and FhuB implies a coordinate activity of both proteins in the transport of iron(III) hydroxamates through the cytoplasmic membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Joshi A. K. Energy coupling in bacterial periplasmic permeases. J Bacteriol. 1990 Aug;172(8):4133–4137. doi: 10.1128/jb.172.8.4133-4137.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Mimura C. S., Shyamala V. Bacterial periplasmic permeases belong to a family of transport proteins operating from Escherichia coli to human: Traffic ATPases. FEMS Microbiol Rev. 1990 Aug;6(4):429–446. doi: 10.1111/j.1574-6968.1990.tb04110.x. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Spurich E. N. Protein-protein interaction in transport: periplasmic histidine-binding protein J interacts with P protein. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1877–1881. doi: 10.1073/pnas.73.6.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerer A., Gaisser S., Braun V. Nucleotide sequences of the sfuA, sfuB, and sfuC genes of Serratia marcescens suggest a periplasmic-binding-protein-dependent iron transport mechanism. J Bacteriol. 1990 Feb;172(2):572–578. doi: 10.1128/jb.172.2.572-578.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K., Köster W., Braun V. Iron(III)hydroxamate transport of Escherichia coli K12: single amino acid replacements at potential ATP-binding sites inactivate the FhuC protein. Mol Gen Genet. 1990 Aug;223(1):159–162. doi: 10.1007/BF00315810. [DOI] [PubMed] [Google Scholar]
- Bell P. E., Nau C. D., Brown J. T., Konisky J., Kadner R. J. Genetic suppression demonstrates interaction of TonB protein with outer membrane transport proteins in Escherichia coli. J Bacteriol. 1990 Jul;172(7):3826–3829. doi: 10.1128/jb.172.7.3826-3829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop L., Agbayani R., Jr, Ambudkar S. V., Maloney P. C., Ames G. F. Reconstitution of a bacterial periplasmic permease in proteoliposomes and demonstration of ATP hydrolysis concomitant with transport. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6953–6957. doi: 10.1073/pnas.86.18.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Gross R., Köster W., Zimmermann L. Plasmid and chromosomal mutants in the iron(III)-aerobactin transport system of Escherichia coli. Use of streptonigrin for selection. Mol Gen Genet. 1983;192(1-2):131–139. doi: 10.1007/BF00327658. [DOI] [PubMed] [Google Scholar]
- Braun V., Günter K., Hantke K. Transport of iron across the outer membrane. Biol Met. 1991;4(1):14–22. doi: 10.1007/BF01135552. [DOI] [PubMed] [Google Scholar]
- Burkhardt R., Braun V. Nucleotide sequence of the fhuC and fhuD genes involved in iron (III) hydroxamate transport: domains in FhuC homologous to ATP-binding proteins. Mol Gen Genet. 1987 Aug;209(1):49–55. doi: 10.1007/BF00329835. [DOI] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Allatt D. D. fhuC and fhuD genes for iron (III)-ferrichrome transport into Escherichia coli K-12. J Bacteriol. 1987 Aug;169(8):3844–3849. doi: 10.1128/jb.169.8.3844-3849.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. L., Nikaido H. Purification and characterization of the membrane-associated components of the maltose transport system from Escherichia coli. J Biol Chem. 1991 May 15;266(14):8946–8951. [PubMed] [Google Scholar]
- Dean D. A., Davidson A. L., Nikaido H. Maltose transport in membrane vesicles of Escherichia coli is linked to ATP hydrolysis. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9134–9138. doi: 10.1073/pnas.86.23.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzandu J. K., Johnson J. F., Wise G. E. Sodium dodecyl sulfate-gel electrophoresis: staining of polypeptides using heavy metal salts. Anal Biochem. 1988 Oct;174(1):157–167. doi: 10.1016/0003-2697(88)90531-3. [DOI] [PubMed] [Google Scholar]
- Eick-Helmerich K., Hantke K., Braun V. Cloning and expression of the exbB gene of Escherichia coli K-12. Mol Gen Genet. 1987 Feb;206(2):246–251. doi: 10.1007/BF00333580. [DOI] [PubMed] [Google Scholar]
- Fecker L., Braun V. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J Bacteriol. 1983 Dec;156(3):1301–1314. doi: 10.1128/jb.156.3.1301-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Günter K., Braun V. Involvement of ExbB and TonB in transport across the outer membrane of Escherichia coli: phenotypic complementation of exb mutants by overexpressed tonB and physical stabilization of TonB by ExbB. J Bacteriol. 1989 Sep;171(9):5127–5134. doi: 10.1128/jb.171.9.5127-5134.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H., Fischer E., Schwarz H., Braun V. Overproduction of the proFhuA outer membrane receptor protein of Escherichia coli K-12: isolation, properties, and immunocytochemical localization at the inner side of the cytoplasmic membrane. Arch Microbiol. 1986 Sep;145(4):334–341. doi: 10.1007/BF00470867. [DOI] [PubMed] [Google Scholar]
- Kerppola R. E., Shyamala V. K., Klebba P., Ames G. F. The membrane-bound proteins of periplasmic permeases form a complex. Identification of the histidine permease HisQMP complex. J Biol Chem. 1991 May 25;266(15):9857–9865. [PubMed] [Google Scholar]
- Köster W., Braun V. Iron (III) hydroxamate transport into Escherichia coli. Substrate binding to the periplasmic FhuD protein. J Biol Chem. 1990 Dec 15;265(35):21407–21410. [PubMed] [Google Scholar]
- Köster W., Braun V. Iron hydroxamate transport of Escherichia coli: nucleotide sequence of the fhuB gene and identification of the protein. Mol Gen Genet. 1986 Sep;204(3):435–442. doi: 10.1007/BF00331021. [DOI] [PubMed] [Google Scholar]
- Köster W., Braun V. Iron(III) hydroxamate transport of Escherichia coli: restoration of iron supply by coexpression of the N- and C-terminal halves of the cytoplasmic membrane protein FhuB cloned on separate plasmids. Mol Gen Genet. 1990 Sep;223(3):379–384. doi: 10.1007/BF00264443. [DOI] [PubMed] [Google Scholar]
- Köster W., Braun V. Iron-hydroxamate transport into Escherichia coli K12: localization of FhuD in the periplasm and of FhuB in the cytoplasmic membrane. Mol Gen Genet. 1989 Jun;217(2-3):233–239. doi: 10.1007/BF02464886. [DOI] [PubMed] [Google Scholar]
- Köster W. Iron(III) hydroxamate transport across the cytoplasmic membrane of Escherichia coli. Biol Met. 1991;4(1):23–32. doi: 10.1007/BF01135553. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Prossnitz E. Determination of a region of the HisJ binding protein involved in the recognition of the membrane complex of the histidine transport system of Salmonella typhimurium. J Biol Chem. 1991 May 25;266(15):9673–9677. [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. Purification and properties of C 55 -isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972 Aug 25;247(16):5123–5131. [PubMed] [Google Scholar]
- Speiser D. M., Ames G. F. Salmonella typhimurium histidine periplasmic permease mutations that allow transport in the absence of histidine-binding proteins. J Bacteriol. 1991 Feb;173(4):1444–1451. doi: 10.1128/jb.173.4.1444-1451.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenmaier H., Van Hove B., Yaraghi Z., Braun V. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J Bacteriol. 1989 May;171(5):2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]