Abstract
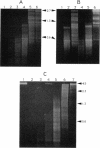
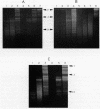
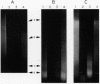
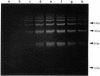
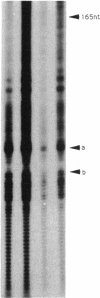
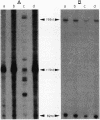
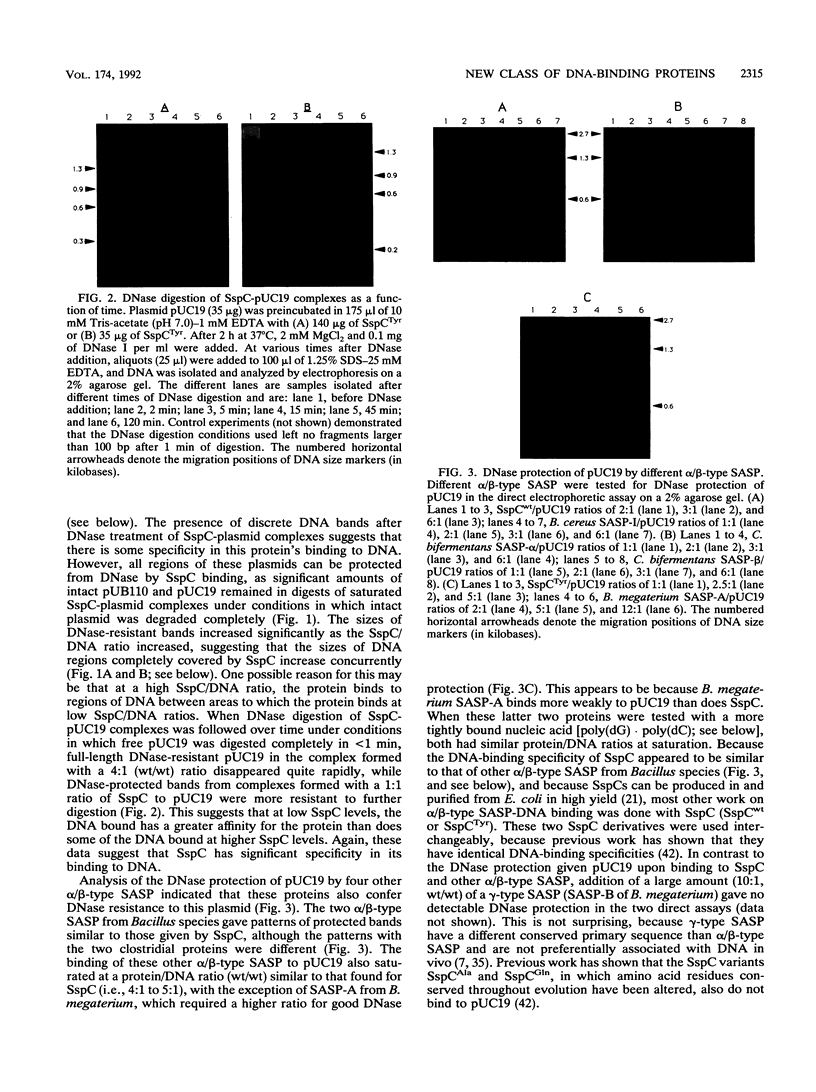
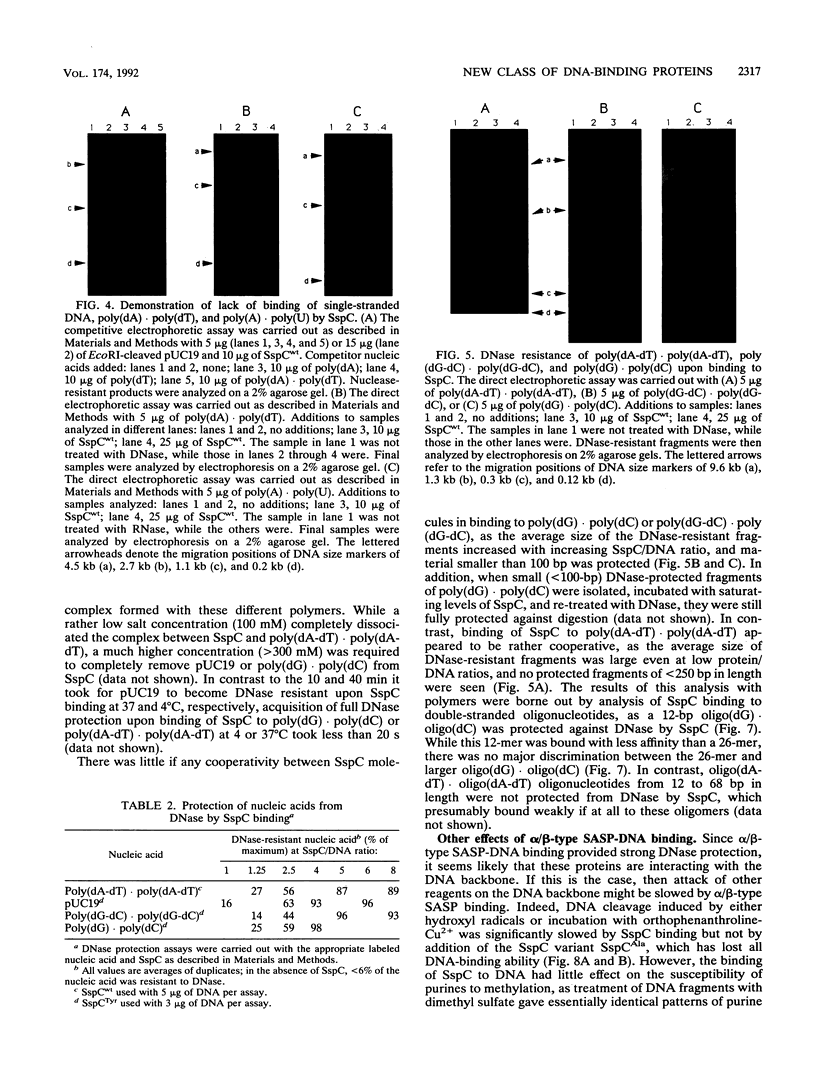
DNA in spores of Bacillus and Clostridium species is associated with small, acid-soluble proteins (SASP) of the alpha/beta type; the presence of these proteins is a major factor in causing spore resistance to UV light, alpha/beta-type SASP did not bind to single-stranded DNA, single- or double-stranded RNA, or DNA-RNA hybrids in vitro. However, these proteins bound a variety of double-stranded DNAs and conferred protection against DNase cleavage. The binding of alpha/beta-type SASP to DNA saturated at a protein/DNA ratio (wt/wt) of 4:1 to 5:1, which is approximately 1 SASP per 4 bp. alpha/beta-type SASP-DNA interaction did not require divalent cations, was independent of pH between 6 and 8, and, for some SASP-DNA pairs, was relatively insensitive to salt up to 0.3 M. The relative affinity of alpha/beta-type SASP for different DNAs was poly(dG).poly(dC) greater than poly(dG-dC).poly(dG-dC) greater than plasmid pUC19 greater than poly(dA-dT).poly(dA-dT), with poly(dA).poly(dT) giving no detectable binding. This order in alpha/beta-type SASP-DNA affinities parallels the facility with which the DNAs adopt an A-like conformation, the conformation in alpha/beta-type SASP-DNA complexes. An oligo(dG).oligo(dC) of 12 bp was bound by alpha/beta-type SASP. While a 26-bp oligo(dG).oligo(dC) bound more tightly than the 12-mer, there was no significant increase in affinity for alpha/beta-type SASP with further increase in size of oligo(dG).oligo(dC). In contrast, binding of alpha/beta-type SASP to oligo(dA-dT).oligo(dA-dT) was minimal up to at least a 70-mer, and binding to poly(dA-dT).poly(dA-dT) was very cooperative. In addition to blocking DNase digestion, binding of alpha/beta-type SASP to DNA blocked (i) cleavage of the DNA backbone by hydroxyl radicals and orthophenanthroline-Cu2+, (ii) DNA cleavage by restriction enzymes, in particular those with specificity for GC-rich sequences; and (iii) in vitro transcription of some but not all genes. However, methylation of dG residues by dimethyl sulfate was not affected by alpha/beta-type SASP binding.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Selsing E. Letter: The structure of polydeoxyguanylic acid with polydeoxycytidylic acid. J Mol Biol. 1974 Sep 15;88(2):551–552. doi: 10.1016/0022-2836(74)90502-6. [DOI] [PubMed] [Google Scholar]
- Arnott S., Selsing E. Structures for the polynucleotide complexes poly(dA) with poly (dT) and poly(dT) with poly(dA) with poly (dT). J Mol Biol. 1974 Sep 15;88(2):509–521. doi: 10.1016/0022-2836(74)90498-7. [DOI] [PubMed] [Google Scholar]
- Becker M. M., Wang Z. B----A transitions within a 5 S ribosomal RNA gene are highly sequence-specific. J Biol Chem. 1989 Mar 5;264(7):4163–4167. [PubMed] [Google Scholar]
- Cabrera-Martinez R. M., Mason J. M., Setlow B., Waites W. M., Setlow P. Purification and amino acid sequence of two small, acid-soluble proteins from Clostridium bifermentans spores. FEMS Microbiol Lett. 1989 Oct 1;52(1-2):139–143. doi: 10.1016/0378-1097(89)90185-7. [DOI] [PubMed] [Google Scholar]
- Cabrera-Martinez R. M., Setlow P. Cloning and nucleotide sequence of three genes coding for small, acid-soluble proteins of Clostridium perfringens spores. FEMS Microbiol Lett. 1991 Jan 15;61(2-3):127–131. doi: 10.1016/0378-1097(91)90539-m. [DOI] [PubMed] [Google Scholar]
- Feavers I. M., Foulkes J., Setlow B., Sun D., Nicholson W., Setlow P., Moir A. The regulation of transcription of the gerA spore germination operon of Bacillus subtilis. Mol Microbiol. 1990 Feb;4(2):275–282. doi: 10.1111/j.1365-2958.1990.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Francesconi S. C., MacAlister T. J., Setlow B., Setlow P. Immunoelectron microscopic localization of small, acid-soluble spore proteins in sporulating cells of Bacillus subtilis. J Bacteriol. 1988 Dec;170(12):5963–5967. doi: 10.1128/jb.170.12.5963-5967.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudibande S. R., Jayasena S. D., Behe M. J. CD studies of double-stranded polydeoxynucleotides composed of repeating units of contiguous homopurine residues. Biopolymers. 1988 Dec;27(12):1905–1915. doi: 10.1002/bip.360271205. [DOI] [PubMed] [Google Scholar]
- Jayasena V. K., Behe M. J. Oligopurine.oligopyrimidine tracts do not have the same conformation as analogous polypurine.polypyrimidines. Biopolymers. 1991 Apr;31(5):511–518. doi: 10.1002/bip.360310506. [DOI] [PubMed] [Google Scholar]
- Magill N. G., Loshon C. A., Setlow P. Small, acid-soluble, spore proteins and their genes from two species of Sporosarcina. FEMS Microbiol Lett. 1990 Nov;60(3):293–297. doi: 10.1016/0378-1097(90)90320-p. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Setlow P. Different small, acid-soluble proteins of the alpha/beta type have interchangeable roles in the heat and UV radiation resistance of Bacillus subtilis spores. J Bacteriol. 1987 Aug;169(8):3633–3637. doi: 10.1128/jb.169.8.3633-3637.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Setlow P. Essential role of small, acid-soluble spore proteins in resistance of Bacillus subtilis spores to UV light. J Bacteriol. 1986 Jul;167(1):174–178. doi: 10.1128/jb.167.1.174-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCall M., Brown T., Kennard O. The crystal structure of d(G-G-G-G-C-C-C-C). A model for poly(dG).poly(dC). J Mol Biol. 1985 Jun 5;183(3):385–396. doi: 10.1016/0022-2836(85)90009-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S. C., Sokolov N. V., He C. M., Setlow P. Binding of small acid-soluble spore proteins from Bacillus subtilis changes the conformation of DNA from B to A. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):77–81. doi: 10.1073/pnas.88.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Nicholson W. L., Setlow B., Setlow P. Binding of DNA in vitro by a small, acid-soluble spore protein from Bacillus subtilis and the effect of this binding on DNA topology. J Bacteriol. 1990 Dec;172(12):6900–6906. doi: 10.1128/jb.172.12.6900-6906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Setlow B., Setlow P. Ultraviolet irradiation of DNA complexed with alpha/beta-type small, acid-soluble proteins from spores of Bacillus or Clostridium species makes spore photoproduct but not thymine dimers. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8288–8292. doi: 10.1073/pnas.88.19.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Setlow P. Dramatic increase in negative superhelicity of plasmid DNA in the forespore compartment of sporulating cells of Bacillus subtilis. J Bacteriol. 1990 Jan;172(1):7–14. doi: 10.1128/jb.172.1.7-14.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Sun D. X., Setlow B., Setlow P. Promoter specificity of sigma G-containing RNA polymerase from sporulating cells of Bacillus subtilis: identification of a group of forespore-specific promoters. J Bacteriol. 1989 May;171(5):2708–2718. doi: 10.1128/jb.171.5.2708-2718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Torigoe C., Tsuboi M. An A-form poly(dG).poly(dC) in H2O solution. Biopolymers. 1985 Sep;24(9):1841–1844. doi: 10.1002/bip.360240913. [DOI] [PubMed] [Google Scholar]
- Panzer S., Losick R., Sun D., Setlow P. Evidence for an additional temporal class of gene expression in the forespore compartment of sporulating Bacillus subtilis. J Bacteriol. 1989 Jan;171(1):561–564. doi: 10.1128/jb.171.1.561-564.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Salas J. L., Santiago-Lara M. L., Setlow B., Sussman M. D., Setlow P. Properties of Bacillus megaterium and Bacillus subtilis mutants which lack the protease that degrades small, acid-soluble proteins during spore germination. J Bacteriol. 1992 Feb;174(3):807–814. doi: 10.1128/jb.174.3.807-814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Hand A. R., Setlow P. Synthesis of a Bacillus subtilis small, acid-soluble spore protein in Escherichia coli causes cell DNA to assume some characteristics of spore DNA. J Bacteriol. 1991 Mar;173(5):1642–1653. doi: 10.1128/jb.173.5.1642-1653.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Localization of low-molecular-weight basic proteins in Bacillus megaterium spores by cross-linking with ultraviolet light. J Bacteriol. 1979 Aug;139(2):486–494. doi: 10.1128/jb.139.2.486-494.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Thymine-containing dimers as well as spore photoproducts are found in ultraviolet-irradiated Bacillus subtilis spores that lack small acid-soluble proteins. Proc Natl Acad Sci U S A. 1987 Jan;84(2):421–423. doi: 10.1073/pnas.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Purification and properties of some unique low molecular weight basic proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8168–8173. [PubMed] [Google Scholar]
- Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- Sigman D. S., Spassky A., Rimsky S., Buc H. Conformational analysis of lac promoters using the nuclease activity of 1,10-phenanthroline-copper ion. Biopolymers. 1985 Jan;24(1):183–197. doi: 10.1002/bip.360240115. [DOI] [PubMed] [Google Scholar]
- Sun D. X., Setlow P. Cloning, nucleotide sequence, and expression of the Bacillus subtilis ans operon, which codes for L-asparaginase and L-aspartase. J Bacteriol. 1991 Jun;173(12):3831–3845. doi: 10.1128/jb.173.12.3831-3845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Stragier P., Setlow P. Identification of a new sigma-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 1989 Feb;3(2):141–149. doi: 10.1101/gad.3.2.141. [DOI] [PubMed] [Google Scholar]
- Sussman M. D., Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis gpr gene, which codes for the protease that initiates degradation of small, acid-soluble proteins during spore germination. J Bacteriol. 1991 Jan;173(1):291–300. doi: 10.1128/jb.173.1.291-300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Rojo F., Setlow P. Effects of mutant small, acid-soluble spore proteins from Bacillus subtilis on DNA in vivo and in vitro. J Bacteriol. 1991 Aug;173(15):4827–4835. doi: 10.1128/jb.173.15.4827-4835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullius T. D., Dombroski B. A. Hydroxyl radical "footprinting": high-resolution information about DNA-protein contacts and application to lambda repressor and Cro protein. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5469–5473. doi: 10.1073/pnas.83.15.5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K., Johnson W. C., Tipper D. J., Setlow P. Comparison of various properties of low-molecular-weight proteins from dormant spores of several Bacillus species. J Bacteriol. 1981 Jun;146(3):965–971. doi: 10.1128/jb.146.3.965-971.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]