Abstract
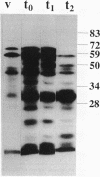
Protein phosphorylation in Bacillus subtilis was assayed in vitro by using extracts prepared from cells at various times during growth and sporulation. At least six proteins were labeled in vitro by using [gamma-32P]ATP and extracts of vegetative cells. In extracts prepared at the end of exponential growth and during stationary phase, 12 to 13 proteins were labeled. Seven of the phosphoproteins were purified by fast-performance liquid chromatography and polyacrylamide gel electrophoresis, blotted to Immobilon membranes, and subjected to partial protein sequencing. One of the sequences had sequence homology (greater than 45%) to elongation factor G from several bacterial species, and four sequences matched the predicted amino-terminal sequences of the outB, orfY-tsr, orfU, and ptsH genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Galizzi A. The Bacillus subtilis outB gene is highly homologous to an Escherichia coli ntr-like gene. J Bacteriol. 1990 Sep;172(9):5482–5485. doi: 10.1128/jb.172.9.5482-5485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbulys D., Trach K. A., Hoch J. A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991 Feb 8;64(3):545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Enami M., Ishihama A. Protein phosphorylation in Escherichia coli and purification of a protein kinase. J Biol Chem. 1984 Jan 10;259(1):526–533. [PubMed] [Google Scholar]
- Janssen G. M., Maessen G. D., Amons R., Möller W. Phosphorylation of elongation factor 1 beta by an endogenous kinase affects its catalytic nucleotide exchange activity. J Biol Chem. 1988 Aug 15;263(23):11063–11066. [PubMed] [Google Scholar]
- Kobayashi H., Kobayashi K., Kobayashi Y. Isolation and characterization of fusidic acid-resistant, sporulation-defective mutants of Bacillus subtilis. J Bacteriol. 1977 Oct;132(1):262–269. doi: 10.1128/jb.132.1.262-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler E., Antranikian G. Covalent modification of proteins in Bacillus subtilis during the process of sporulation, germination and outgrowth. FEMS Microbiol Lett. 1989 Jan 1;48(1):87–92. doi: 10.1016/0378-1097(89)90152-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mitchell C., Vary J. C. Proteins that interact with GTP during sporulation of Bacillus subtilis. J Bacteriol. 1989 Jun;171(6):2915–2918. doi: 10.1128/jb.171.6.2915-2918.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Sutrina S. L., Saier M. H., Stewart G. C., Peterkofsky A., Reddy P. Mechanistic and physiological consequences of HPr(ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J. 1989 Jul;8(7):2111–2120. doi: 10.1002/j.1460-2075.1989.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Kada T. Formation of competent Bacillus subtilis cells. J Bacteriol. 1983 Feb;153(2):813–821. doi: 10.1128/jb.153.2.813-821.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Wu L. F., Reizer J. Regulation of bacterial physiological processes by three types of protein phosphorylating systems. Trends Biochem Sci. 1990 Oct;15(10):391–395. doi: 10.1016/0968-0004(90)90238-7. [DOI] [PubMed] [Google Scholar]
- Shay L. K., Vary J. C. Biochemical studies on glucose initiated germination in Bacillus megaterium. Biochim Biophys Acta. 1978 Jan 18;538(2):284–292. doi: 10.1016/0304-4165(78)90356-2. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Stock A. M., Mottonen J. M. Signal transduction in bacteria. Nature. 1990 Mar 29;344(6265):395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- Trach K., Chapman J. W., Piggot P., LeCoq D., Hoch J. A. Complete sequence and transcriptional analysis of the spo0F region of the Bacillus subtilis chromosome. J Bacteriol. 1988 Sep;170(9):4194–4208. doi: 10.1128/jb.170.9.4194-4208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Koshland D. E., Jr The identification of distinct protein kinases and phosphatases in the prokaryote Salmonella typhimurium. J Biol Chem. 1981 May 10;256(9):4640–4648. [PubMed] [Google Scholar]
- Ying C. W., Ordal G. W. Nucleotide sequence and expression of cheF, an essential gene for chemotaxis in Bacillus subtilis. J Bacteriol. 1989 Mar;171(3):1631–1637. doi: 10.1128/jb.171.3.1631-1637.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]