Abstract
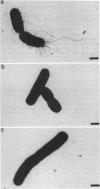
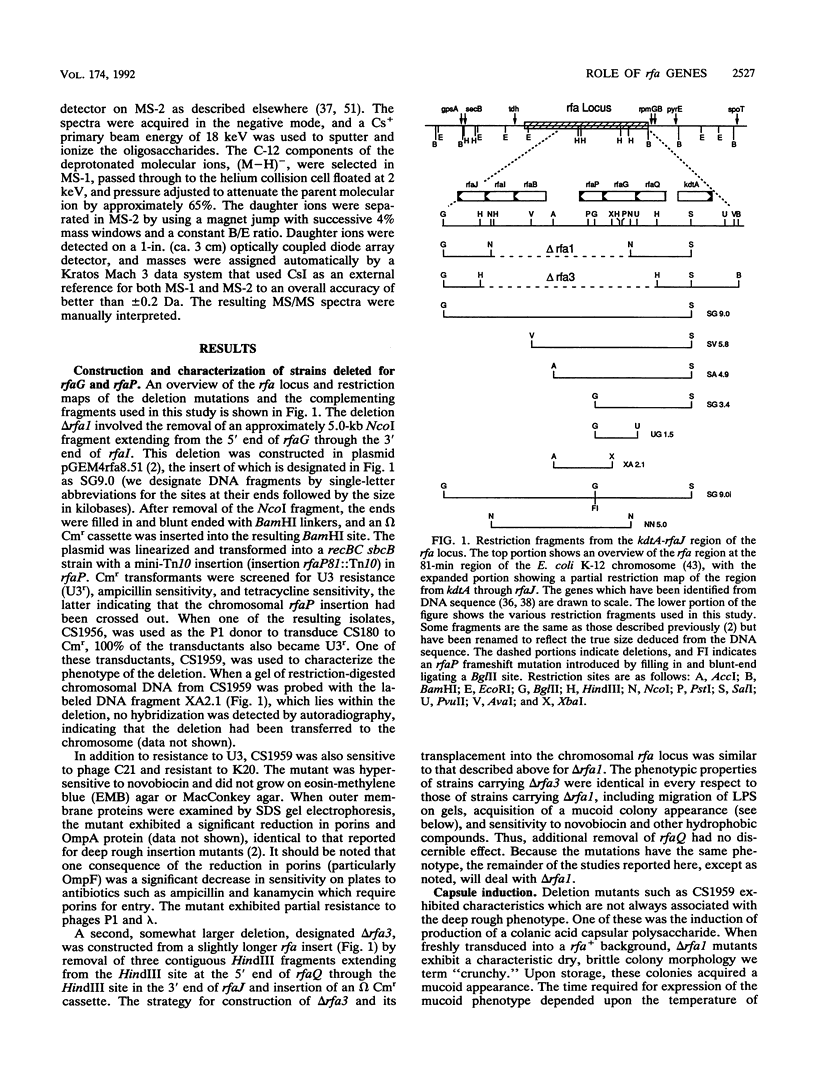
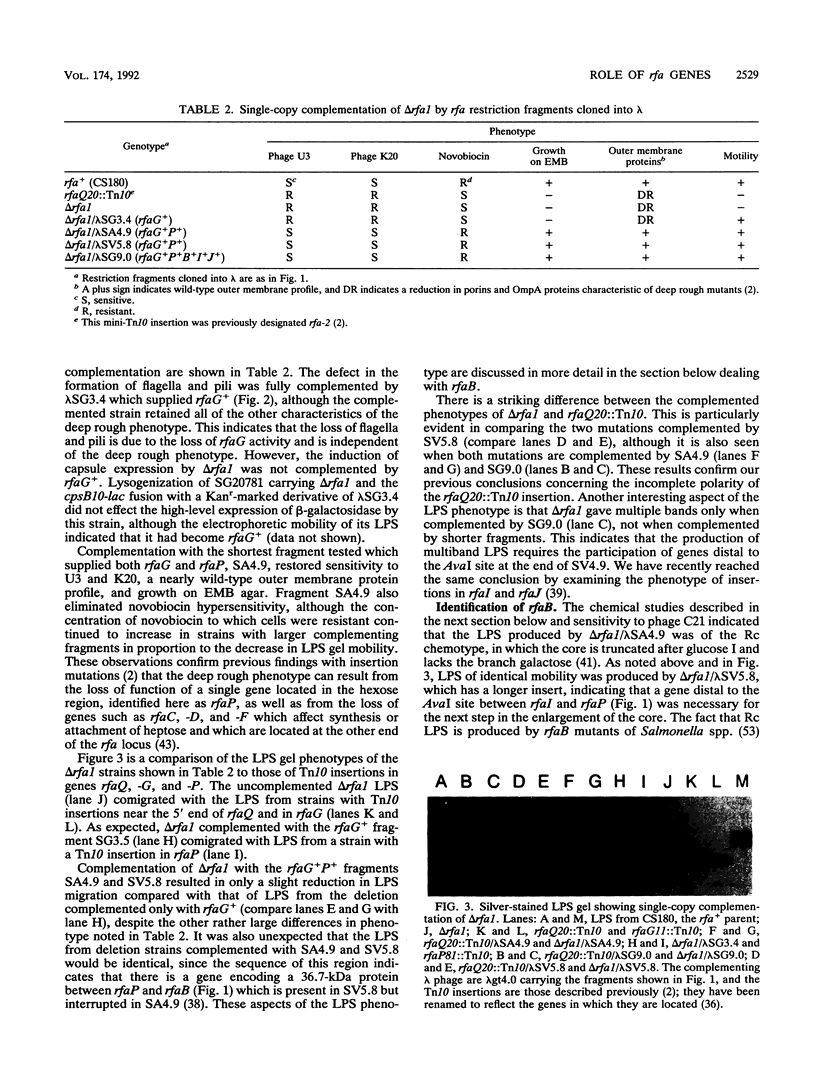
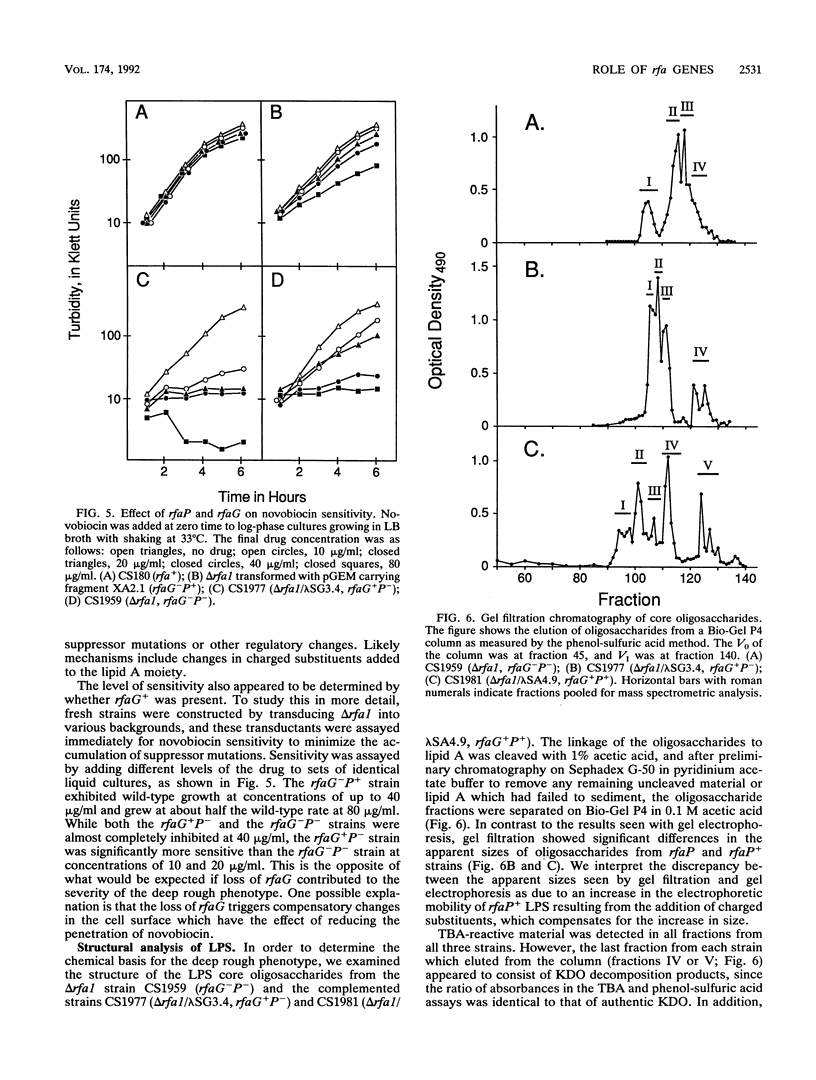
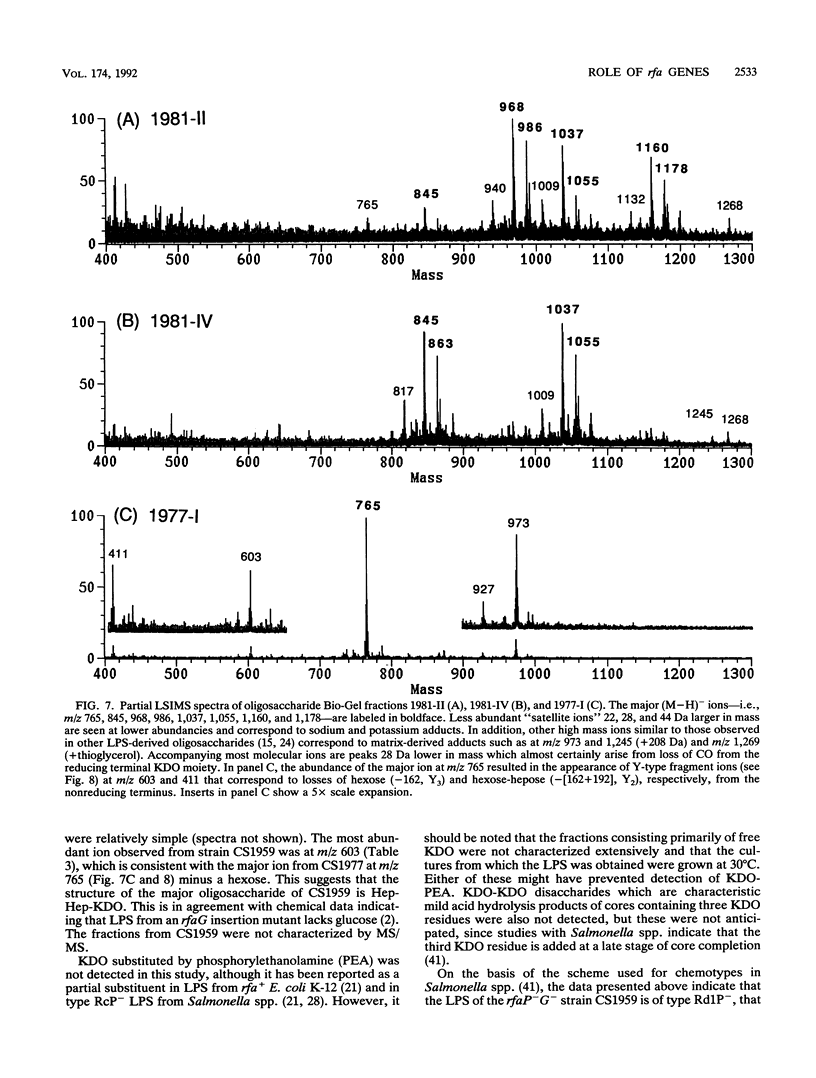
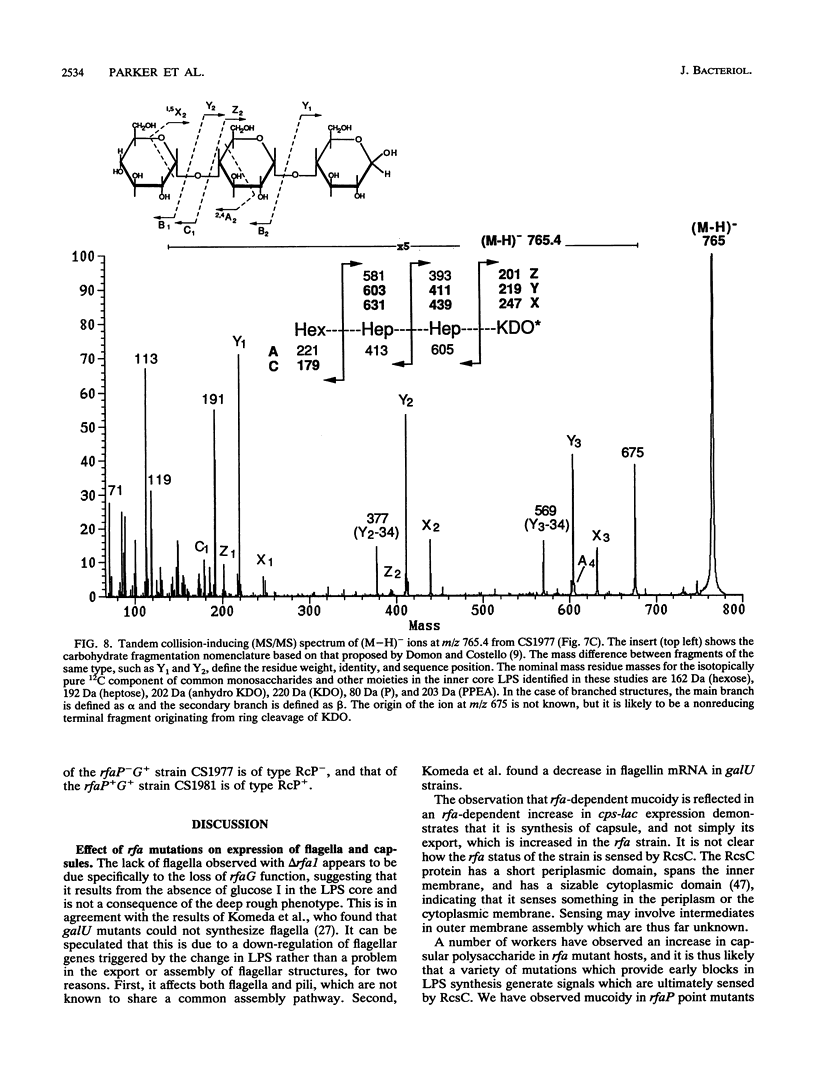
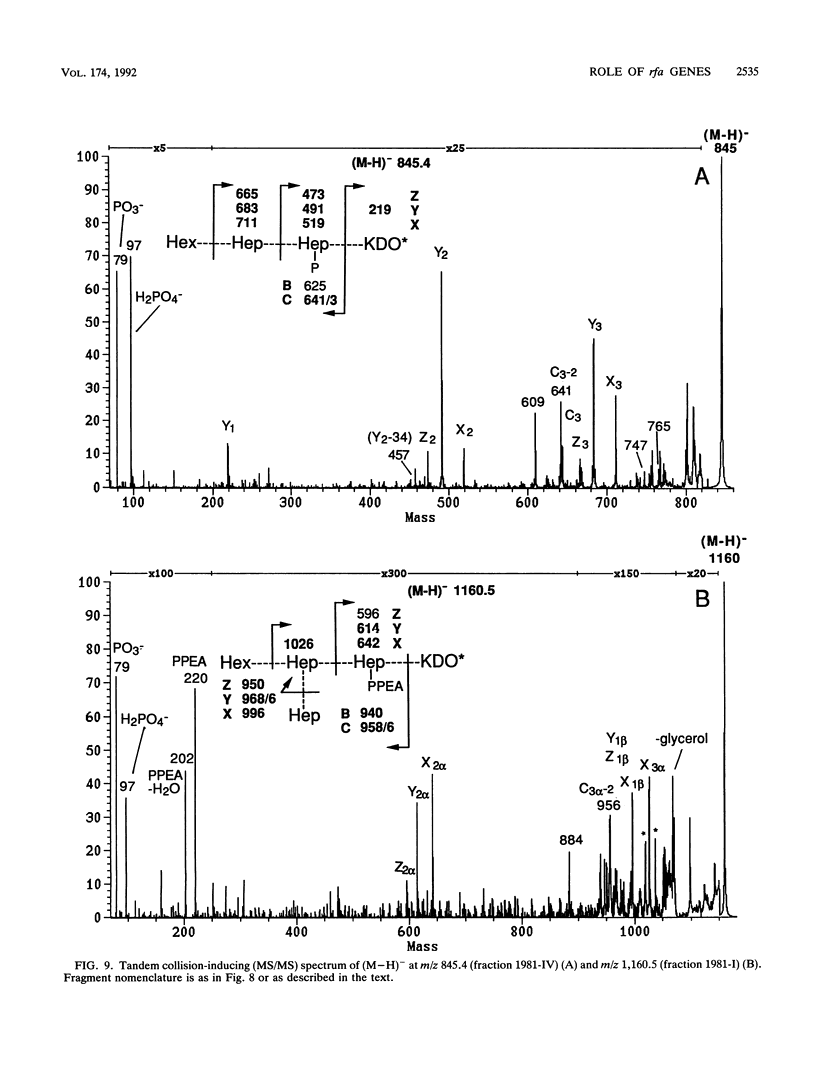
Deletions which removed rfa genes involved in lipopolysaccharide (LPS) core synthesis were constructed in vitro and inserted into the chromosome by linear transformation. The deletion delta rfa1, which removed rfaGPBI, resulted in a truncated LPS core containing two heptose residues but no hexose and a deep rought phenotype including decreased expression of major outer membrane proteins, hypersensitivity to novobiocin, and resistance to phage U3. In addition, delta rfa1 resulted in the loss of flagella and pili and a mucoid colony morphology. Measurement of the synthesis of beta-galactosidase from a cps-lacZ fusion showed that the mucoid phenotype was due to rcsC-dependent induction of colanic acid capsular polysaccharide synthesis. Complementation of delta rfa1 with rfaG+ DNA fragments resulted in a larger core and restored the synthesis of flagella and pili but did not reverse the deep rough phenotype or the induction of cps-lacZ, while complementation with a fragment carrying only rfaP+ reversed the deep rough phenotype but not the loss of flagella and pili. A longer deletion which removed rfaQGPBIJ was also constructed, and complementation studies with this deletion showed that the product of rfaQ was not required for the functions of rfaG and rfaP. Thus, the function of rfaQ remains unknown. Tandem mass spectrometric analysis of LPS core oligosaccharides from complemented delta rfa1 strains indicated that rfaP+ was necessary for the addition of either phosphoryl (P) or pyrophosphorylethanolamine (PPEA) substituents to the heptose I residue, as well as for the partial branch substitution of heptose II by heptose III. The substitution of heptose II is independent of the type of P substituent present on heptose I, and this results in four different core structures. A model is presented which relates the deep rough phenotype to the loss of heptose-linked P and PPEA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin E. A., Graves J. F., Hite L. A., Parker C. T., Schnaitman C. A. Genetic analysis of lipopolysaccharide core biosynthesis by Escherichia coli K-12: insertion mutagenesis of the rfa locus. J Bacteriol. 1990 Sep;172(9):5312–5325. doi: 10.1128/jb.172.9.5312-5325.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M. G., Schnaitman C. A. Regulation of the OmpA outer membrane protein of Escherichia coli. J Bacteriol. 1981 Sep;147(3):972–985. doi: 10.1128/jb.147.3.972-985.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill J. A., Quinlan-Walshe C., Gottesman S. Fine-structure mapping and identification of two regulators of capsule synthesis in Escherichia coli K-12. J Bacteriol. 1988 Jun;170(6):2599–2611. doi: 10.1128/jb.170.6.2599-2611.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstenius P., Flock J. I., Lindberg A. Nucleotide sequence of rfaI and rfaJ genes encoding lipopolysaccharide glycosyl transferases from Salmonella typhimurium. Nucleic Acids Res. 1990 Oct 25;18(20):6128–6128. doi: 10.1093/nar/18.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catron K. M., Schnaitman C. A. Export of protein in Escherichia coli: a novel mutation in ompC affects expression of other major outer membrane proteins. J Bacteriol. 1987 Sep;169(9):4327–4334. doi: 10.1128/jb.169.9.4327-4334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Click E. M., McDonald G. A., Schnaitman C. A. Translational control of exported proteins that results from OmpC porin overexpression. J Bacteriol. 1988 May;170(5):2005–2011. doi: 10.1128/jb.170.5.2005-2011.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich D. L., Stein M. A., Schnaitman C. A. Associations of Escherichia coli K-12 OmpF trimers with rough and smooth lipopolysaccharides. J Bacteriol. 1990 Sep;172(9):5307–5311. doi: 10.1128/jb.172.9.5307-5311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W., Ruschmann E., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants. 4. Phosphate groups linked to heptose units and their absence in some R lipopolysaccharides. Eur J Biochem. 1968 Mar;4(1):134–138. doi: 10.1111/j.1432-1033.1968.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Falick A. M., Wang G. H., Walls F. C. Ion source for liquid matrix secondary ionization mass spectrometry. Anal Chem. 1986 Jun;58(7):1308–1311. doi: 10.1021/ac00298a009. [DOI] [PubMed] [Google Scholar]
- Fellay R., Frey J., Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52(2-3):147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gibson B. W., Webb J. W., Yamasaki R., Fisher S. J., Burlingame A. L., Mandrell R. E., Schneider H., Griffiss J. M. Structure and heterogeneity of the oligosaccharides from the lipopolysaccharides of a pyocin-resistant Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1989 Jan;86(1):17–21. doi: 10.1073/pnas.86.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Trisler P., Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985 Jun;162(3):1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin M., Kennedy E. P. Role of phosphatidylethanolamine in the biosynthesis of pyrophosphoethanolamine residues in the lipopolysaccharide of Escherichia coli. J Biol Chem. 1982 Nov 10;257(21):12475–12477. [PubMed] [Google Scholar]
- Helander I. M., Vaara M., Sukupolvi S., Rhen M., Saarela S., Zähringer U., Mäkelä P. H. rfaP mutants of Salmonella typhimurium. Eur J Biochem. 1989 Nov 20;185(3):541–546. doi: 10.1111/j.1432-1033.1989.tb15147.x. [DOI] [PubMed] [Google Scholar]
- Hodgson A. L., Bird P., Nisbet I. T. Cloning, nucleotide sequence, and expression in Escherichia coli of the phospholipase D gene from Corynebacterium pseudotuberculosis. J Bacteriol. 1990 Mar;172(3):1256–1261. doi: 10.1128/jb.172.3.1256-1261.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst O., Röhrscheidt-Andrzejewski E., Brade H. Isolation and characterisation of 3-deoxy-D-manno-2-octulopyranosonate 7-(2-aminoethyl phosphate) from the inner core region of Escherichia coli K-12 and Salmonella minnesota lipopolysaccharides. Carbohydr Res. 1990 Sep 5;204:93–102. doi: 10.1016/0008-6215(90)84024-o. [DOI] [PubMed] [Google Scholar]
- Hämmerling G., Lehmann V., Lüderitz O. Structural studies on the heptose region of Salmonella lipopolysaccharides. Eur J Biochem. 1973 Oct 18;38(3):453–458. doi: 10.1111/j.1432-1033.1973.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Ingham C., Buechner M., Adler J. Effect of outer membrane permeability on chemotaxis in Escherichia coli. J Bacteriol. 1990 Jul;172(7):3577–3583. doi: 10.1128/jb.172.7.3577-3583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson P. E., Lindberg A. A., Lindberg B., Wollin R. Structural studies on the hexose region of the core in lipopolysaccharides from Enterobacteriaceae. Eur J Biochem. 1981 Apr;115(3):571–577. doi: 10.1111/j.1432-1033.1981.tb06241.x. [DOI] [PubMed] [Google Scholar]
- John C. M., Griffiss J. M., Apicella M. A., Mandrell R. E., Gibson B. W. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J Biol Chem. 1991 Oct 15;266(29):19303–19311. [PubMed] [Google Scholar]
- Kadam S. K., Rehemtulla A., Sanderson K. E. Cloning of rfaG, B, I, and J genes for glycosyltransferase enzymes for synthesis of the lipopolysaccharide core of Salmonella typhimurium. J Bacteriol. 1985 Jan;161(1):277–284. doi: 10.1128/jb.161.1.277-284.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Komeda Y., Icho T., Iino T. Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol. 1977 Feb;129(2):908–915. doi: 10.1128/jb.129.2.908-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann V., Lüderitz O., Westphal O. The linkage of pyrophosphorylethanolamine to heptose in the core of Salmonella minnesota lipopolysaccharides. Eur J Biochem. 1971 Aug 16;21(3):339–347. doi: 10.1111/j.1432-1033.1971.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Leive L. An equilibrium between two fractions of lipopolysaccharide in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1435–1439. doi: 10.1073/pnas.61.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlradt P. F. Biosynthesis of Salmonella lipopolysaccharide. Studies on the transfer of glucose, galactose, and phosphate to the core in a cell free system. Eur J Biochem. 1971 Jan 1;18(1):20–27. doi: 10.1111/j.1432-1033.1971.tb01209.x. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Golecki J. R. Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur J Biochem. 1975 Feb 21;51(2):343–352. doi: 10.1111/j.1432-1033.1975.tb03934.x. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. Biosynthesis of Salmonella lipopolysaccharide. The in vitro transfer of phosphate to the heptose moiety of the core. Eur J Biochem. 1969 Dec;11(2):241–248. doi: 10.1111/j.1432-1033.1969.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. T., Pradel E., Schnaitman C. A. Identification and sequences of the lipopolysaccharide core biosynthetic genes rfaQ, rfaP, and rfaG of Escherichia coli K-12. J Bacteriol. 1992 Feb;174(3):930–934. doi: 10.1128/jb.174.3.930-934.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips N. J., John C. M., Reinders L. G., Gibson B. W., Apicella M. A., Griffiss J. M. Structural models for the cell surface lipooligosaccharides of Neisseria gonorrhoeae and Haemophilus influenzae. Biomed Environ Mass Spectrom. 1990 Nov;19(11):731–745. doi: 10.1002/bms.1200191112. [DOI] [PubMed] [Google Scholar]
- Pradel E., Schnaitman C. A. Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J Bacteriol. 1991 Oct;173(20):6428–6431. doi: 10.1128/jb.173.20.6428-6431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P., Stirm S., Jann B., Jann K. Cell-wall lipopolysaccharide from Escherichia coli B. Eur J Biochem. 1975 Aug 1;56(1):41–55. doi: 10.1111/j.1432-1033.1975.tb02205.x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A., Parker C. T., Klena J. D., Pradel E. L., Pearson N. B., Sanderson K. E., MacClachlan P. R. Physical maps of the rfa loci of Escherichia coli K-12 and Salmonella typhimurium. J Bacteriol. 1991 Dec;173(23):7410–7411. doi: 10.1128/jb.173.23.7410-7411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen K., Nikaido H. Lipopolysaccharide structure required for in vitro trimerization of Escherichia coli OmpF porin. J Bacteriol. 1991 Jan;173(2):926–928. doi: 10.1128/jb.173.2.926-928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman J. A., Benson S. A. Bacteriophage K20 requires both the OmpF porin and lipopolysaccharide for receptor function. J Bacteriol. 1987 Oct;169(10):4830–4833. doi: 10.1128/jb.169.10.4830-4833.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout V., Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990 Feb;172(2):659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout V., Torres-Cabassa A., Maurizi M. R., Gutnick D., Gottesman S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J Bacteriol. 1991 Mar;173(5):1738–1747. doi: 10.1128/jb.173.5.1738-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain S. M., Armitage I. M., Anderson L., Takayama K., Qureshi N., Raetz C. R. Location of polar substituents and fatty acyl chains on lipid A precursors from a 3-deoxy-D-manno-octulosonic acid-deficient mutant of Salmonella typhimurium. Studies by 1H, 13C, and 31P nuclear magnetic resonance. J Biol Chem. 1985 Dec 25;260(30):16089–16098. [PubMed] [Google Scholar]
- Trisler P., Gottesman S. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1984 Oct;160(1):184–191. doi: 10.1128/jb.160.1.184-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollin R., Creeger E. S., Rothfield L. I., Stocker B. A., Lindberg A. A. Salmonella typhimurium mutants defective in UDP-D-galactose:lipopolysaccharide alpha 1,6-D-galactosyltransferase. Structural, immunochemical, and enzymologic studies of rfaB mutants. J Biol Chem. 1983 Mar 25;258(6):3769–3774. [PubMed] [Google Scholar]