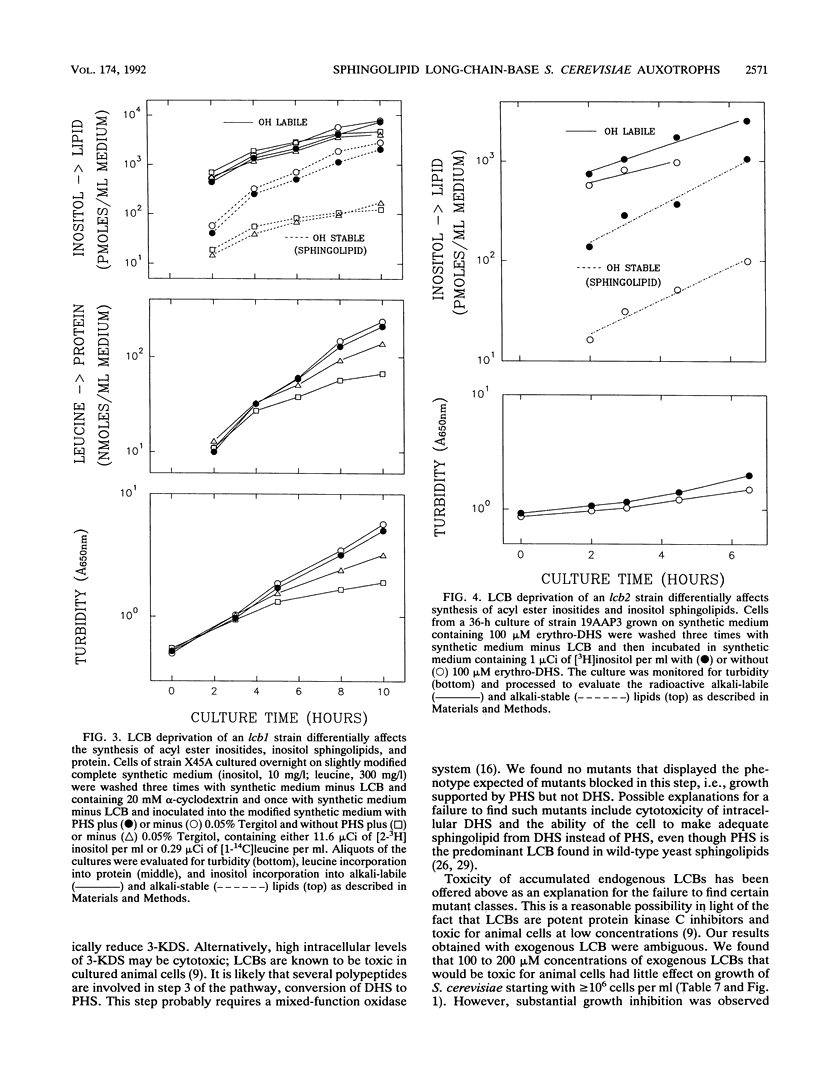
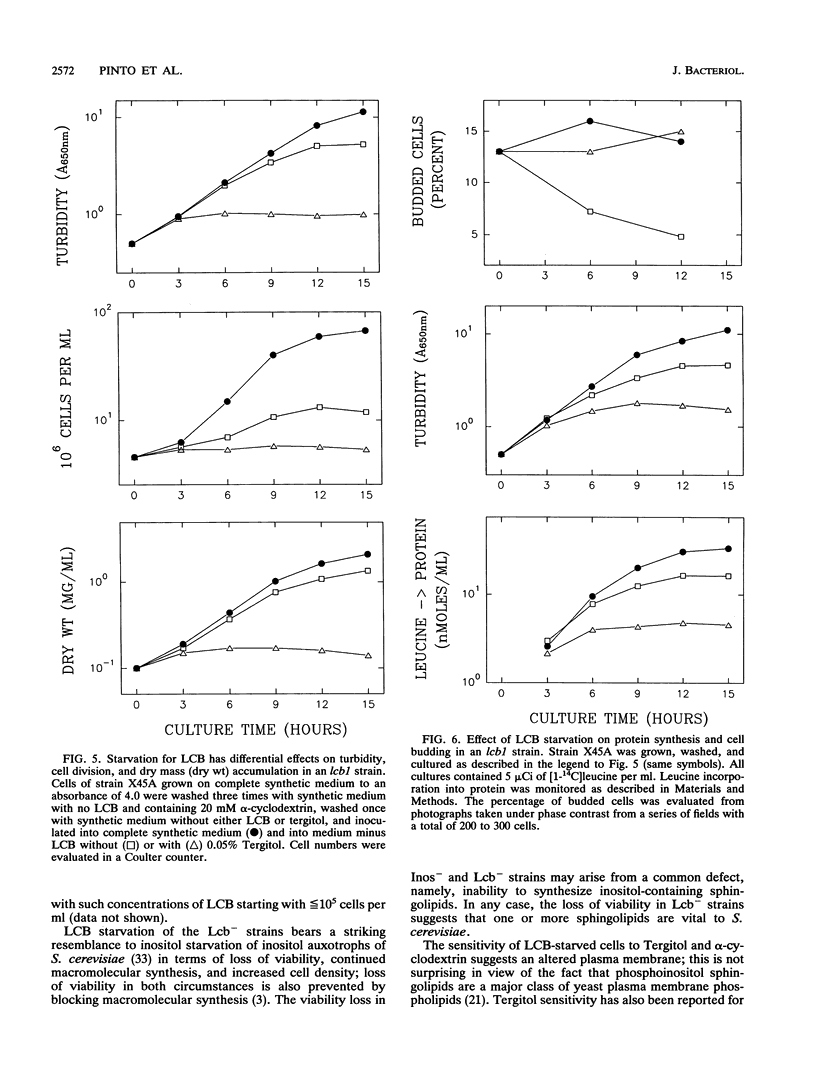
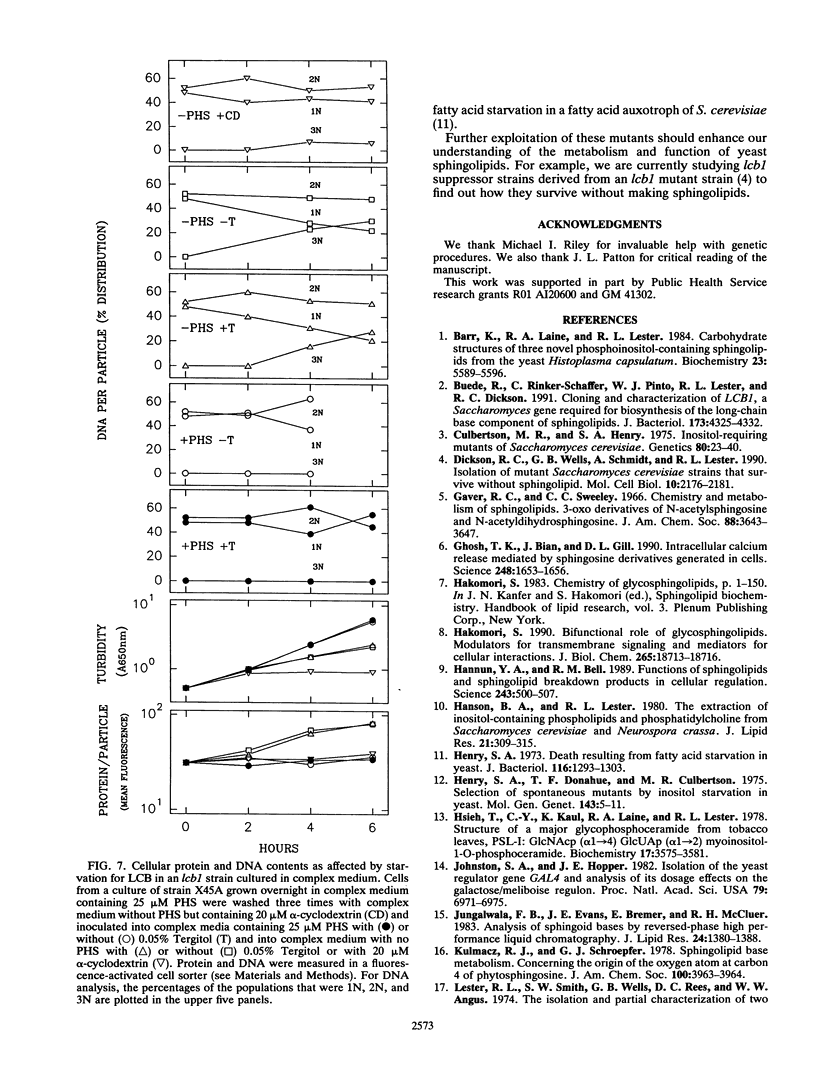
Abstract
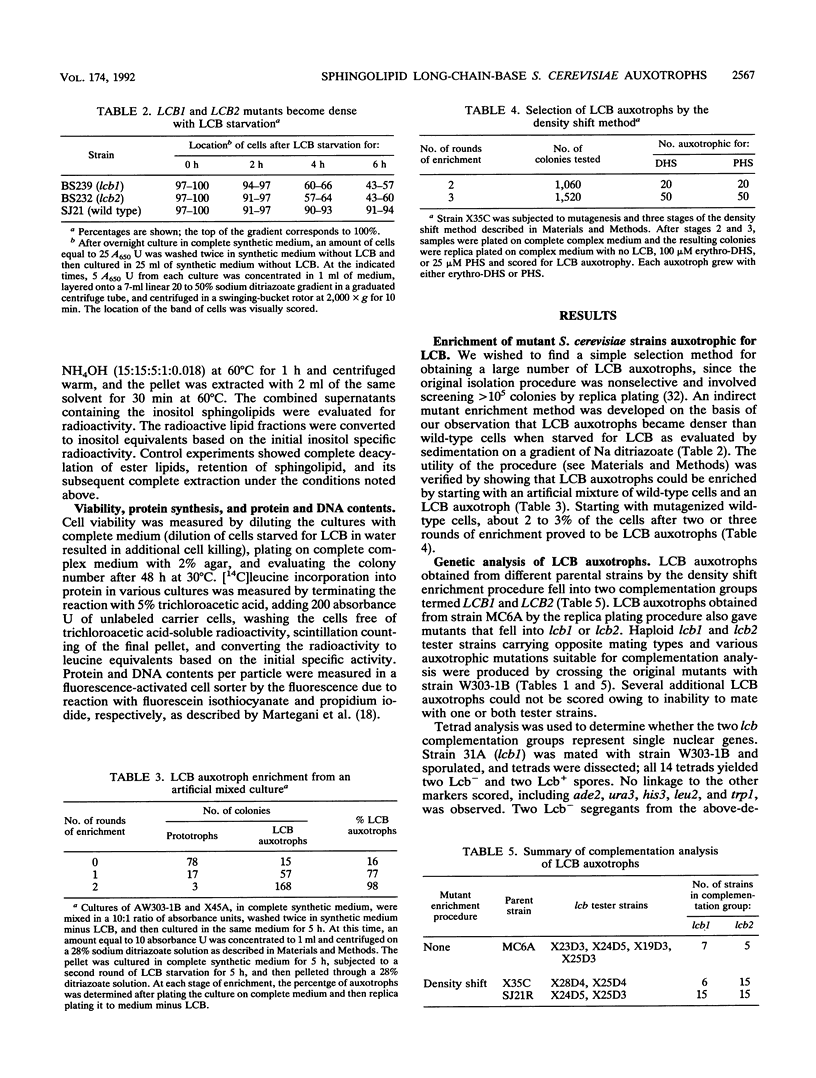
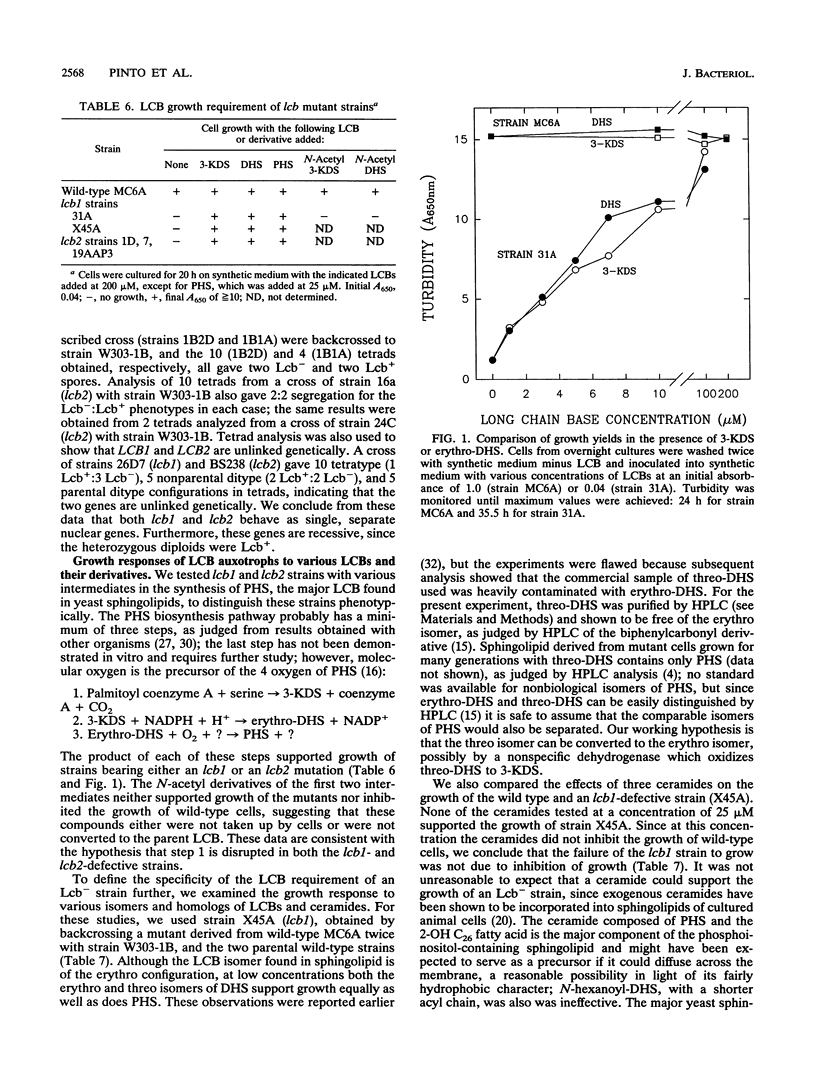
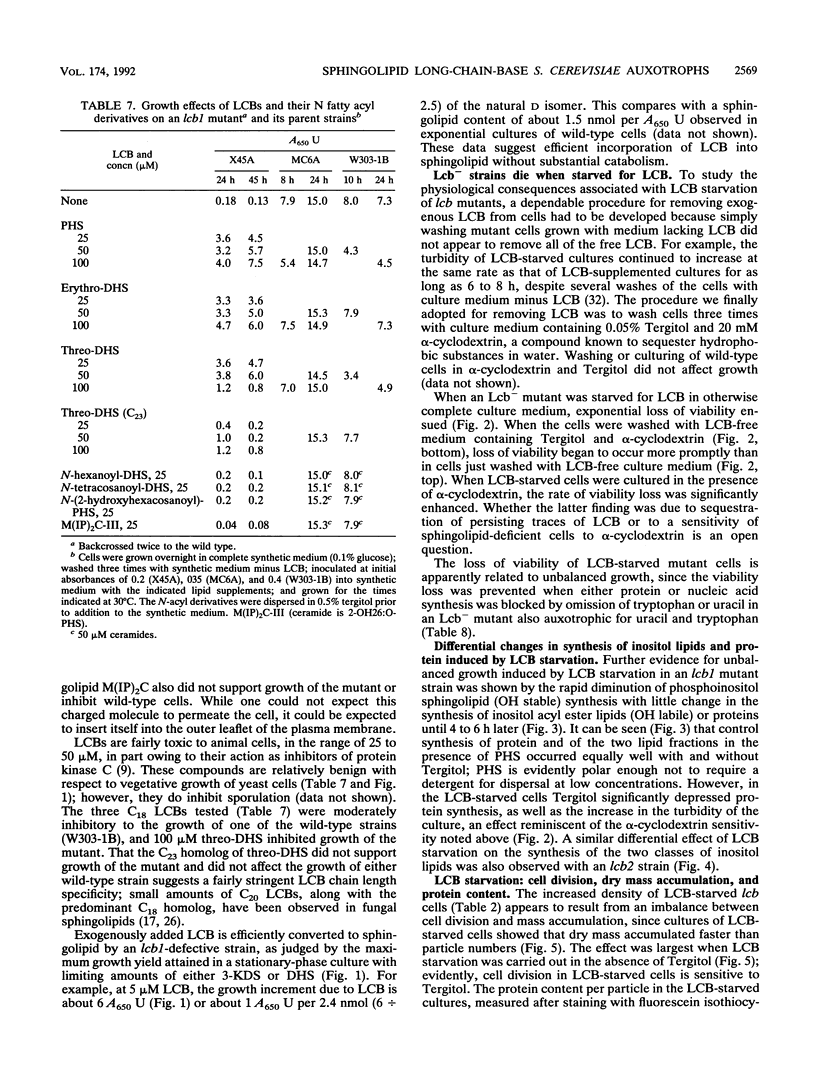
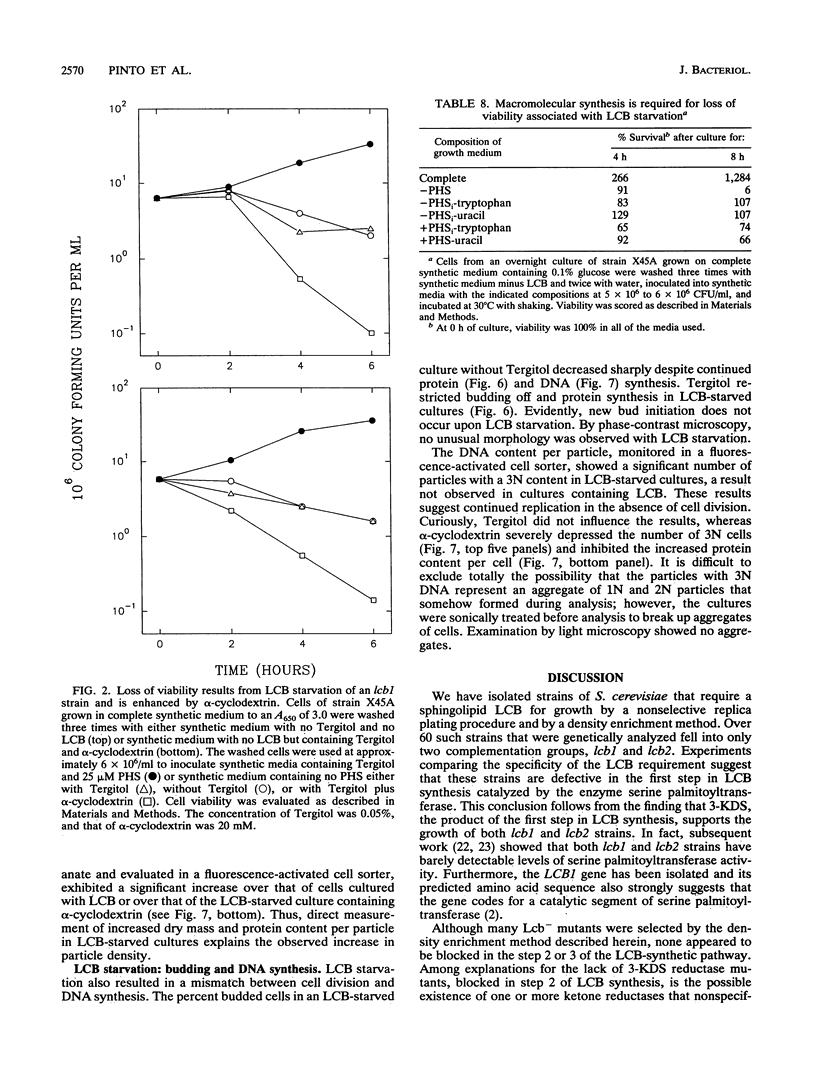
A selection method for sphingolipid long-chain-base auxotrophs of Saccharomyces cerevisiae was devised after observing that strains that require a long-chain base for growth become denser when starved for this substance. Genetic analysis of over 60 such strains indicated only two complementation classes, lcb1 and lcb2. Mutant strains from each class grew equally well with 3-ketodihydrosphingosine, erythrodihydrosphingosine or threodihydrosphingosine, or phytosphingosine. Since these metabolites represent the first, second, and last components, respectively, of the long-chain-base biosynthetic pathway, it is likely that the LCB1 and LCB2 genes are involved in the first step of long-chain-base synthesis. The results of long-chain-base starvation in the Lcb- strains suggest that one or more sphingolipids have a vital role in S. cerevisiae. Immediate sequelae of long-chain-base starvation were loss of viability, exacerbated in the presence of alpha-cyclodextrin, and loss of phosphoinositol sphingolipid synthesis but not phosphatidylinositol synthesis. Loss of viability with long-chain-base starvation could be prevented by also blocking either protein or nucleic acid synthesis. Without a long-chain-base, cell division, dry mass accumulation, and protein synthesis continued at a diminished rate and were further inhibited by the detergent Tergitol. The cell density increase induced by long-chain-base starvation is thus explained as a differential loss of cell division and mass accumulation. Long-chain-base starvation in Lcb- S. cerevisiae and inositol starvation of Inos- S. cerevisiae share common features: an increase in cell density and a loss of cell viability overcome by blocking macromolecular synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr K., Laine R. A., Lester R. L. Carbohydrate structures of three novel phosphoinositol-containing sphingolipids from the yeast Histoplasma capsulatum. Biochemistry. 1984 Nov 6;23(23):5589–5596. doi: 10.1021/bi00318a032. [DOI] [PubMed] [Google Scholar]
- Buede R., Rinker-Schaffer C., Pinto W. J., Lester R. L., Dickson R. C. Cloning and characterization of LCB1, a Saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids. J Bacteriol. 1991 Jul;173(14):4325–4332. doi: 10.1128/jb.173.14.4325-4332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Wells G. B., Schmidt A., Lester R. L. Isolation of mutant Saccharomyces cerevisiae strains that survive without sphingolipids. Mol Cell Biol. 1990 May;10(5):2176–2181. doi: 10.1128/mcb.10.5.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaver R. C., Sweeley C. C. Chemistry and metabolism of sphingolipids. 3-Oxo derivatives of N-acetylsphingosine and N-acetyldihydrosphingosine. J Am Chem Soc. 1966 Aug 5;88(15):3643–3647. doi: 10.1021/ja00967a032. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J., Gill D. L. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990 Jun 29;248(4963):1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hanson B. A., Lester R. L. The extraction of inositol-containing phospholipids and phosphatidylcholine from Saccharomyces cerevisiae and Neurospora crassa. J Lipid Res. 1980 Mar;21(3):309–315. [PubMed] [Google Scholar]
- Henry S. A. Death resulting from fatty acid starvation in yeast. J Bacteriol. 1973 Dec;116(3):1293–1303. doi: 10.1128/jb.116.3.1293-1303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Donahue T. F., Culbertson M. R. Selection of spontaneous mutants by inositol starvation in yeast. Mol Gen Genet. 1975 Dec 30;143(1):5–11. doi: 10.1007/BF00269415. [DOI] [PubMed] [Google Scholar]
- Hsieh T. C., Kaul K., Laine R. A., Lester R. L. Structure of a major glycophosphoceramide from tobacco leaves, PSL-I: 2-deoxy-2-acetamido-D-glucopyranosyl(alpha1 leads to 4)-D-glucuronopyranosyl(alpha1 leads to 2)myoinositol-1-O-phosphoceramide. Biochemistry. 1978 Aug 22;17(17):3575–3581. doi: 10.1021/bi00610a024. [DOI] [PubMed] [Google Scholar]
- Johnston S. A., Hopper J. E. Isolation of the yeast regulatory gene GAL4 and analysis of its dosage effects on the galactose/melibiose regulon. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6971–6975. doi: 10.1073/pnas.79.22.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungalwala F. B., Evans J. E., Bremer E., McCluer R. H. Analysis of sphingoid bases by reversed-phase high performance liquid chromatography. J Lipid Res. 1983 Oct;24(10):1380–1388. [PubMed] [Google Scholar]
- Lester R. L., Smith S. W., Wells G. B., Rees D. C., Angus W. W. The isolation and partial characterization of two novel sphingolipids from Neurospora crassa: di(inositolphosphoryl)ceramide and ((gal)3glu)ceramide. J Biol Chem. 1974 Jun 10;249(11):3388–3394. [PubMed] [Google Scholar]
- Martegani E., Vanoni M., Delia D. A computer algorithm for the analysis of protein distribution in budding yeast. Cytometry. 1984 Jan;5(1):81–85. doi: 10.1002/cyto.990050112. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Martin O. C. A series of fluorescent N-acylsphingosines: synthesis, physical properties, and studies in cultured cells. Biochemistry. 1988 Jun 14;27(12):4439–4445. doi: 10.1021/bi00412a034. [DOI] [PubMed] [Google Scholar]
- Patton J. L., Lester R. L. The phosphoinositol sphingolipids of Saccharomyces cerevisiae are highly localized in the plasma membrane. J Bacteriol. 1991 May;173(10):3101–3108. doi: 10.1128/jb.173.10.3101-3108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto W. J., Wells G. W., Lester R. L. Characterization of enzymatic synthesis of sphingolipid long-chain bases in Saccharomyces cerevisiae: mutant strains exhibiting long-chain-base auxotrophy are deficient in serine palmitoyltransferase activity. J Bacteriol. 1992 Apr;174(8):2575–2581. doi: 10.1128/jb.174.8.2575-2581.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. W., Lester R. L. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J Biol Chem. 1974 Jun 10;249(11):3395–3405. [PubMed] [Google Scholar]
- Snell E. E., Dimari S. J., Brady R. N. Biosynthesis of sphingosine and dihydrosphingosine by cell-free systems from Hansenula ciferri. Chem Phys Lipids. 1970 Oct;5(1):116–138. doi: 10.1016/0009-3084(70)90013-7. [DOI] [PubMed] [Google Scholar]
- Steiner S., Smith S., Waechter C. J., Lester R. L. Isolation and partial characterization of a major inositol-containing lipid in baker's yeast, mannosyl-diinositol, diphosphoryl-ceramide. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1042–1048. doi: 10.1073/pnas.64.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W. Studies on the biosynthesis and degradation of sphingosine bases. Chem Phys Lipids. 1970 Oct;5(1):139–158. doi: 10.1016/0009-3084(70)90014-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Botstein D. Ordered Linear Tetrads Are Produced by the Sporulation of Newly Formed Zygotes of Saccharomyces cerevisiae. Genetics. 1987 Feb;115(2):229–232. doi: 10.1093/genetics/115.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. B., Lester R. L. The isolation and characterization of a mutant strain of Saccharomyces cerevisiae that requires a long chain base for growth and for synthesis of phosphosphingolipids. J Biol Chem. 1983 Sep 10;258(17):10200–10203. [PubMed] [Google Scholar]
- White M. J., Lopes J. M., Henry S. A. Inositol metabolism in yeasts. Adv Microb Physiol. 1991;32:1–51. doi: 10.1016/s0065-2911(08)60004-1. [DOI] [PubMed] [Google Scholar]


