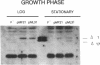
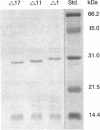
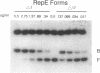
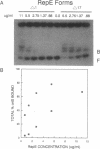
Abstract
Plasmid F replication is controlled by a plasmid-specified Rep protein with both autorepressor and initiator functions. The mechanism by which these two functions of a Rep protein are balanced to achieve stable replication is unknown; however, we speculated in prior work that Rep protein modification could be involved. We report here that naturally proteolyzed F RepE protein has been detected and characterized. The processed molecule lost the first 17 N-terminal aminoacyl residues and initiator function but acquired increased specific DNA-binding affinity in the presence of Escherichia coli chromosomal DNA. When supplied in trans, the altered protein acts as an incompatibility substance and eliminates maintenance of F'lac. These findings indicate that protein processing has the potential to contribute to the overall control of DNA replication.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chattoraj D. K., Mason R. J., Wickner S. H. Mini-P1 plasmid replication: the autoregulation-sequestration paradox. Cell. 1988 Feb 26;52(4):551–557. doi: 10.1016/0092-8674(88)90468-0. [DOI] [PubMed] [Google Scholar]
- Frame R., Bishop J. O. The number of sex-factors per chromosome in Escherichia coli. Biochem J. 1971 Jan;121(1):93–103. doi: 10.1042/bj1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener A., Filutowicz M. S., McEachern M. J., Helinski D. R. N-terminal truncated forms of the bifunctional pi initiation protein express negative activity on plasmid R6K replication. Mol Gen Genet. 1990 Oct;224(1):24–32. doi: 10.1007/BF00259447. [DOI] [PubMed] [Google Scholar]
- Helsberg M., Ebbers J., Eichenlaub R. Mutations affecting replication and copy number control in plasmid mini-F both reside in the gene for the 29-kDa protein. Plasmid. 1985 Jul;14(1):53–63. doi: 10.1016/0147-619x(85)90032-0. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Keasling J. D., Palsson B. O., Cooper S. Cell-cycle-specific F plasmid replication: regulation by cell size control of initiation. J Bacteriol. 1991 Apr;173(8):2673–2680. doi: 10.1128/jb.173.8.2673-2680.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C. A review of mini-F plasmid maintenance. Plasmid. 1985 Jul;14(1):1–16. doi: 10.1016/0147-619x(85)90027-7. [DOI] [PubMed] [Google Scholar]
- Kline B. C. Aspects of plasmid F maintenance in Escherichia coli. Can J Microbiol. 1988 Apr;34(4):526–535. doi: 10.1139/m88-090. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Seelke R. W. Genetic evidence that control of F replication is negative. Mol Gen Genet. 1982;187(2):218–224. doi: 10.1007/BF00331120. [DOI] [PubMed] [Google Scholar]
- Kline B. C., Trawick J. Identification and characterization of a second copy number control gene in mini-F plasmids. Mol Gen Genet. 1983;192(3):408–415. doi: 10.1007/BF00392183. [DOI] [PubMed] [Google Scholar]
- Lane H. E. Replication and incompatibility of F and plasmids in the IncFI Group. Plasmid. 1981 Jan;5(1):100–126. doi: 10.1016/0147-619x(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Method for the isolation of the replication region of a bacterial replicon: construction of a mini-F'kn plasmid. J Bacteriol. 1976 Aug;127(2):982–987. doi: 10.1128/jb.127.2.982-987.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki S., Kuribayashi M., Miki T., Horiuchi T. An amber replication mutant of F plasmid mapped in the minimal replication region. Mol Gen Genet. 1983;191(2):231–237. doi: 10.1007/BF00334819. [DOI] [PubMed] [Google Scholar]
- Manis J. J., Kline B. C. Restriction endonuclease mapping and mutagenesis of the F sex factor replication region. Mol Gen Genet. 1977 Apr 29;152(3):175–182. doi: 10.1007/BF00268815. [DOI] [PubMed] [Google Scholar]
- Masson L., Ray D. S. Mechanism of autonomous control of the Escherichia coli F plasmid: different complexes of the initiator/repressor protein are bound to its operator and to an F plasmid replication origin. Nucleic Acids Res. 1986 Jul 25;14(14):5693–5711. doi: 10.1093/nar/14.14.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson L., Ray D. S. Mechanism of autonomous control of the Escherichia coli F plasmid: purification and characterization of the repE gene product. Nucleic Acids Res. 1988 Jan 25;16(2):413–424. doi: 10.1093/nar/16.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraiso K., Mukhopadhyay G., Chattoraj D. K. Location of a P1 plasmid replication inhibitor determinant within the initiator gene. J Bacteriol. 1990 Aug;172(8):4441–4447. doi: 10.1128/jb.172.8.4441-4447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraiso K., Tokino T., Murotsu T., Matsubara K. Replication of mini-F plasmid in vitro promoted by purified E protein. Mol Gen Genet. 1987 Mar;206(3):519–521. doi: 10.1007/BF00428895. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K., Sugisaki H., Takanami M. Nine unique repeating sequences in a region essential for replication and incompatibility of the mini-F plasmid. Gene. 1981 Nov;15(2-3):257–271. doi: 10.1016/0378-1119(81)90135-9. [DOI] [PubMed] [Google Scholar]
- Rio D. C., Tjian R. SV40 T antigen binding site mutations that affect autoregulation. Cell. 1983 Apr;32(4):1227–1240. doi: 10.1016/0092-8674(83)90305-7. [DOI] [PubMed] [Google Scholar]
- Seelke R. W., Kline B. C., Trawick J. D., Ritts G. D. Genetic studies of F plasmid maintenance genes involved in copy number control, incompatability, and partitioning. Plasmid. 1982 Mar;7(2):163–179. doi: 10.1016/0147-619x(82)90075-0. [DOI] [PubMed] [Google Scholar]
- Sekimizu K., Bramhill D., Kornberg A. ATP activates dnaA protein in initiating replication of plasmids bearing the origin of the E. coli chromosome. Cell. 1987 Jul 17;50(2):259–265. doi: 10.1016/0092-8674(87)90221-2. [DOI] [PubMed] [Google Scholar]
- Sekimizu K., Kornberg A. Cardiolipin activation of dnaA protein, the initiation protein of replication in Escherichia coli. J Biol Chem. 1988 May 25;263(15):7131–7135. [PubMed] [Google Scholar]
- Shields M. S., Kline B. C., Tam J. E. Similarities in control of mini-F plasmid and chromosomal replication in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3375–3378. doi: 10.1128/jb.169.7.3375-3378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Søgaard-Andersen L., Rokeach L. A., Molin S. Regulated expression of a gene important for replication of plasmid F in E. coli. EMBO J. 1984 Feb;3(2):257–262. doi: 10.1002/j.1460-2075.1984.tb01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Nozue H., Zeng H., Hayashi T., Kamio Y., Itoh Y. Effects of mutations in the repA gene of plasmid Rts1 on plasmid replication and autorepressor function. J Bacteriol. 1990 Feb;172(2):786–792. doi: 10.1128/jb.172.2.786-792.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokino T., Murotsu T., Matsubara K. Purification and properties of the mini-F plasmid-encoded E protein needed for autonomous replication control of the plasmid. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4109–4113. doi: 10.1073/pnas.83.12.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Helinski D. R. Separation of the minimal replication region of the F plasmid into a replication origin segment and a trans-acting segment. Mol Gen Genet. 1982;186(3):372–377. doi: 10.1007/BF00729456. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawick J. D., Kline B. C. A two-stage molecular model for control of mini-F replication. Plasmid. 1985 Jan;13(1):59–69. doi: 10.1016/0147-619x(85)90056-3. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Fujiyama A., Murotsu T., Matsubara K. Role of nine repeating sequences of the mini-F genome for expression of F-specific incompatibility phenotype and copy number control. J Bacteriol. 1983 Jul;155(1):337–344. doi: 10.1128/jb.155.1.337-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson L. A., Phua S. H., Bergquist P. L., Lane H. E. An Mr 29000 protein is essential for mini-F maintenance in E. coli. Gene. 1982 Sep;19(2):173–178. doi: 10.1016/0378-1119(82)90003-8. [DOI] [PubMed] [Google Scholar]
- Womble D. D., Rownd R. H. Regulation of mini-F plasmid DNA replication. A quantitative model for control of plasmid mini-F replication in the bacterial cell division cycle. J Mol Biol. 1987 May 5;195(1):99–113. doi: 10.1016/0022-2836(87)90330-5. [DOI] [PubMed] [Google Scholar]