Abstract
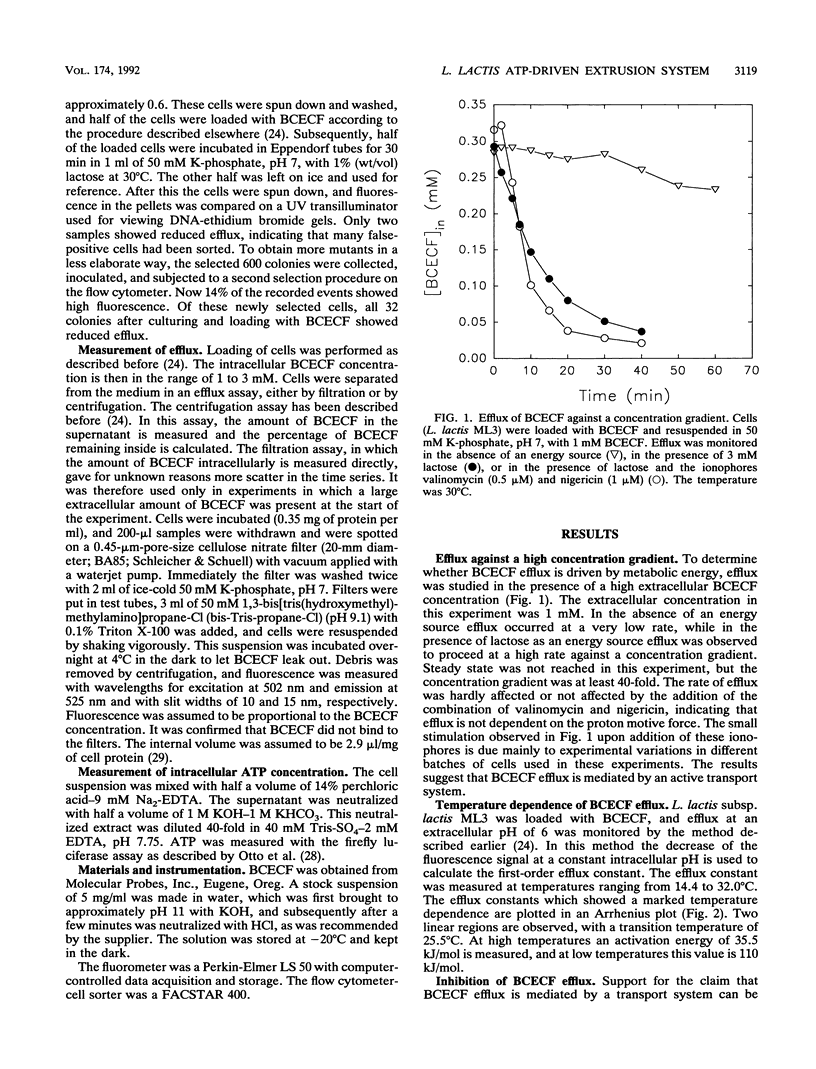
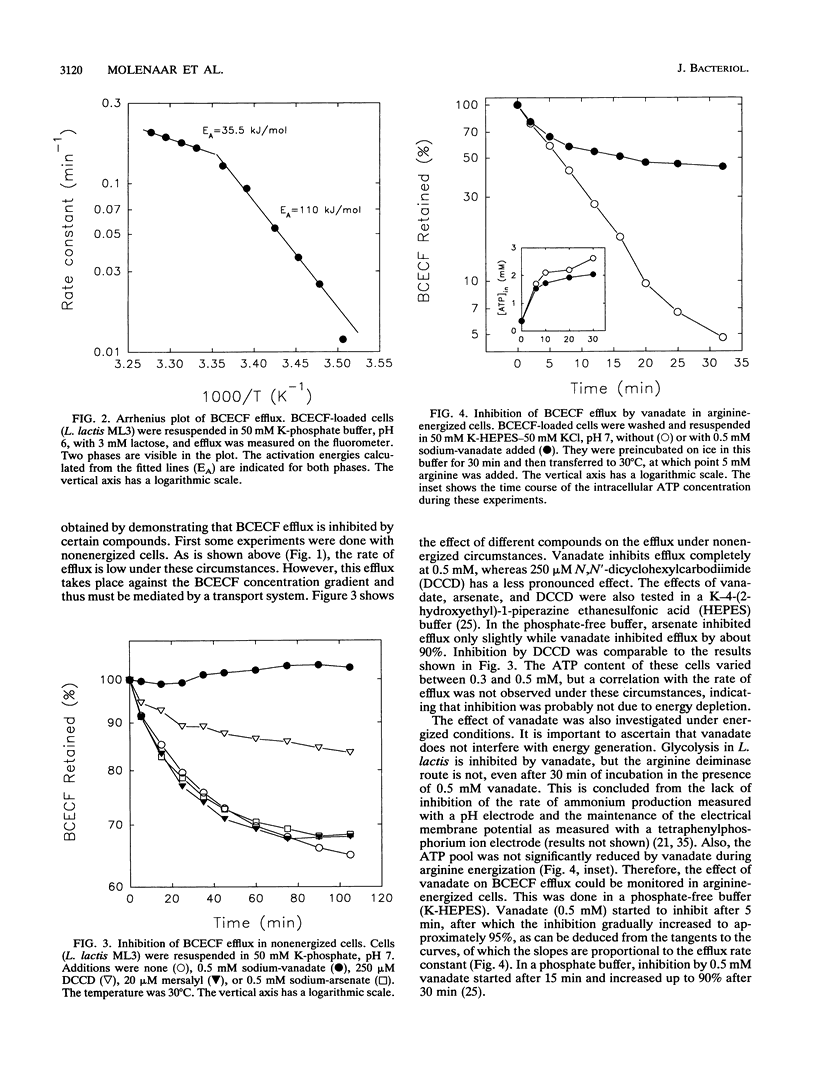
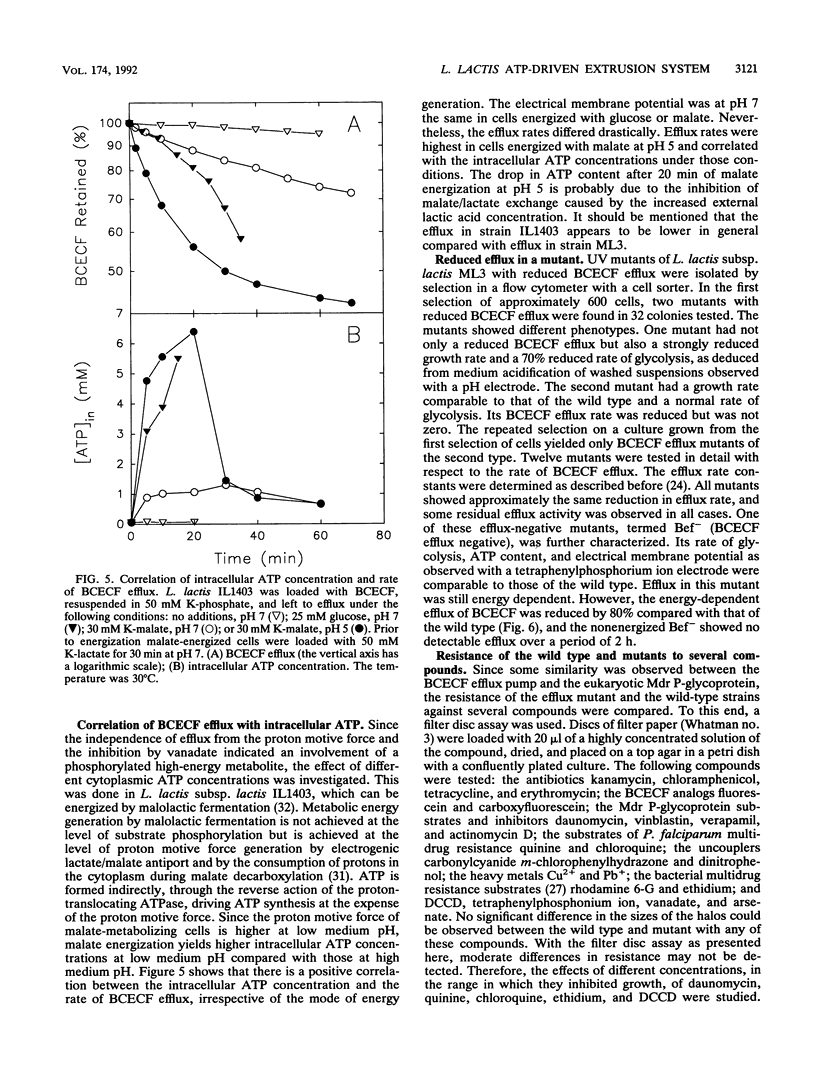
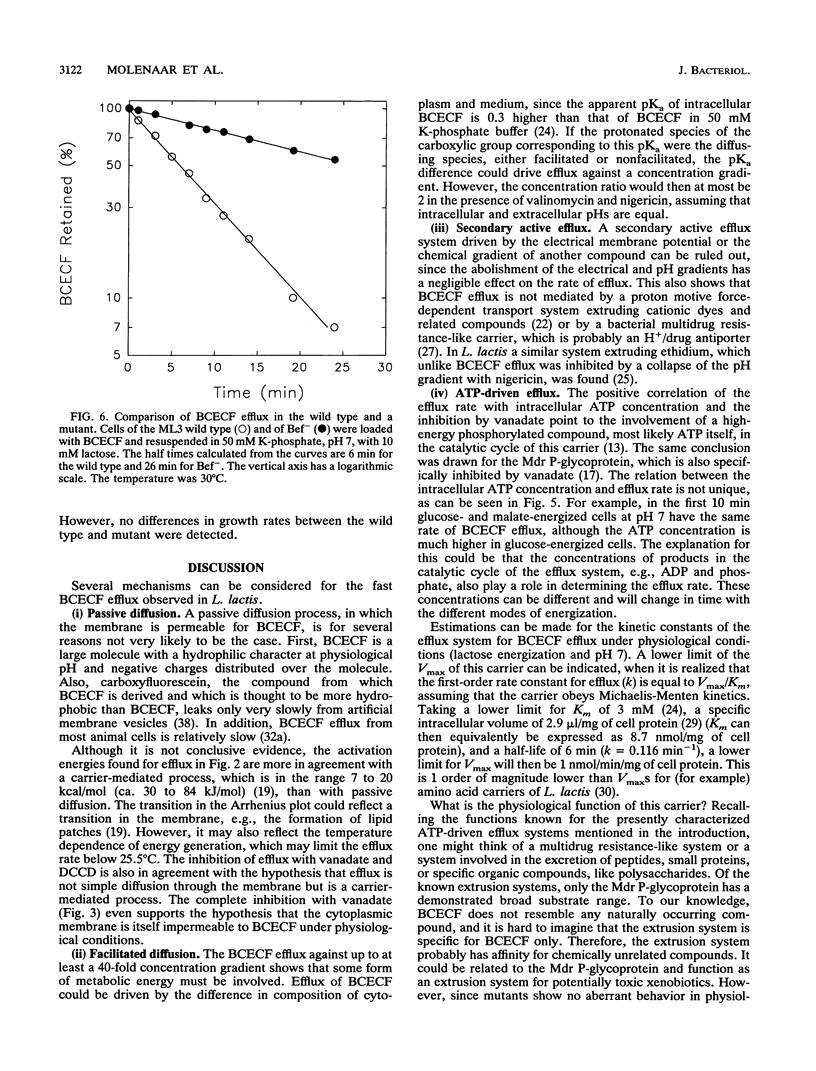
Many bacteria, both gram positive and gram negative, extrude in an energy-dependent manner the fluorescent pH indicator 2',7'-bis-(2-carboxyethyl)-5[and -6]-carboxyfluorescein (BCECF) (D. Molenaar, T. Abee, and W. N. Konings, Biochim. Biophys. Acta 1115:75-83, 1991). This efflux was studied in detail in Lactococcus lactis, and several indications that a transport system is involved were found. This transport system is most likely driven by ATP or a related compound. The evidence is that BCECF extrusion (i) occurs against a BCECF gradient, (ii) is strictly correlated with ATP concentration and not with the proton motive force, and (iii) is inhibited by vanadate and to a lesser extent by N,N'-dicyclohexylcarbodiimide. Most convincingly, a UV mutant with a strongly reduced efflux rate was isolated. Such a mutant was isolated from a BCECF-loaded and lactose-energized population by selection of highly fluorescent cells in a flow cytometer-cell sorter. The physiological function of this extrusion system is unknown, but its characteristics classify it among the traffic ATPases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. N., Harpur E. S., Gray T. J., Simmons N. L., Hirst B. H. Efflux of bis-carboxyethyl-carboxyfluorescein (BCECF) by a novel ATP-dependent transport mechanism in epithelial cells. Biochem Biophys Res Commun. 1990 Oct 15;172(1):262–267. doi: 10.1016/s0006-291x(05)80203-7. [DOI] [PubMed] [Google Scholar]
- Bradley G., Juranka P. F., Ling V. Mechanism of multidrug resistance. Biochim Biophys Acta. 1988 Aug 3;948(1):87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Callahan H. L., Beverley S. M. Heavy metal resistance: a new role for P-glycoproteins in Leishmania. J Biol Chem. 1991 Oct 5;266(28):18427–18430. [PubMed] [Google Scholar]
- Cangelosi G. A., Martinetti G., Leigh J. A., Lee C. C., Thienes C., Theines C., Nester E. W. Role for [corrected] Agrobacterium tumefaciens ChvA protein in export of beta-1,2-glucan. J Bacteriol. 1989 Mar;171(3):1609–1615. doi: 10.1128/jb.171.3.1609-1615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote S. J., Thompson J. K., Cowman A. F., Kemp D. J. Amplification of the multidrug resistance gene in some chloroquine-resistant isolates of P. falciparum. Cell. 1989 Jun 16;57(6):921–930. doi: 10.1016/0092-8674(89)90330-9. [DOI] [PubMed] [Google Scholar]
- Garrido M. C., Herrero M., Kolter R., Moreno F. The export of the DNA replication inhibitor Microcin B17 provides immunity for the host cell. EMBO J. 1988 Jun;7(6):1853–1862. doi: 10.1002/j.1460-2075.1988.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson L., Mahanty H. K., Kolter R. Genetic analysis of an MDR-like export system: the secretion of colicin V. EMBO J. 1990 Dec;9(12):3875–3884. doi: 10.1002/j.1460-2075.1990.tb07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Croop J., Housman D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell. 1986 Nov 7;47(3):371–380. doi: 10.1016/0092-8674(86)90594-5. [DOI] [PubMed] [Google Scholar]
- Guilfoile P. G., Hutchinson C. R. A bacterial analog of the mdr gene of mammalian tumor cells is present in Streptomyces peucetius, the producer of daunorubicin and doxorubicin. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8553–8557. doi: 10.1073/pnas.88.19.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Härtlein M., Schiessl S., Wagner W., Rdest U., Kreft J., Goebel W. Transport of hemolysin by Escherichia coli. J Cell Biochem. 1983;22(2):87–97. doi: 10.1002/jcb.240220203. [DOI] [PubMed] [Google Scholar]
- Létoffé S., Delepelaire P., Wandersman C. Protease secretion by Erwinia chrysanthemi: the specific secretion functions are analogous to those of Escherichia coli alpha-haemolysin. EMBO J. 1990 May;9(5):1375–1382. doi: 10.1002/j.1460-2075.1990.tb08252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgley M. An efflux system for cationic dyes and related compounds in Escherichia coli. Microbiol Sci. 1987 Apr;4(4):125–127. [PubMed] [Google Scholar]
- Mimura C. S., Holbrook S. R., Ames G. F. Structural model of the nucleotide-binding conserved component of periplasmic permeases. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):84–88. doi: 10.1073/pnas.88.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar D., Abee T., Konings W. N. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta. 1991 Nov 14;1115(1):75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- Müller M., Ishikawa T., Berger U., Klünemann C., Lucka L., Schreyer A., Kannicht C., Reutter W., Kurz G., Keppler D. ATP-dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110-kDa glycoprotein binding ATP and bile salt. J Biol Chem. 1991 Oct 5;266(28):18920–18926. [PubMed] [Google Scholar]
- Neyfakh A. A., Bidnenko V. E., Chen L. B. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4781–4785. doi: 10.1073/pnas.88.11.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Hellingwerf K. J., Konings W. N. Regulation of the glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J Bacteriol. 1987 May;169(5):2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Konings W. N. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J Bacteriol. 1988 Feb;170(2):700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault P., Gaillardin C., Heslot H. Role of malolactic fermentation in lactic acid bacteria. Biochimie. 1988 Mar;70(3):375–379. doi: 10.1016/0300-9084(88)90210-6. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. I., Eady E. A., Cove J. H., Cunliffe W. J., Baumberg S., Wootton J. C. Inducible erythromycin resistance in staphylococci is encoded by a member of the ATP-binding transport super-gene family. Mol Microbiol. 1990 Jul;4(7):1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Rosteck P. R., Jr, Reynolds P. A., Hershberger C. L. Homology between proteins controlling Streptomyces fradiae tylosin resistance and ATP-binding transport. Gene. 1991 Jun 15;102(1):27–32. doi: 10.1016/0378-1119(91)90533-h. [DOI] [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Stanfield S. W., Ielpi L., O'Brochta D., Helinski D. R., Ditta G. S. The ndvA gene product of Rhizobium meliloti is required for beta-(1----2)glucan production and has homology to the ATP-binding export protein HlyB. J Bacteriol. 1988 Aug;170(8):3523–3530. doi: 10.1128/jb.170.8.3523-3530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. N., Blumenthal R., Klausner R. D. Carboxyfluorescein leakage assay for lipoprotein-liposome interaction. Methods Enzymol. 1986;128:657–668. doi: 10.1016/0076-6879(86)28098-2. [DOI] [PubMed] [Google Scholar]


