Abstract
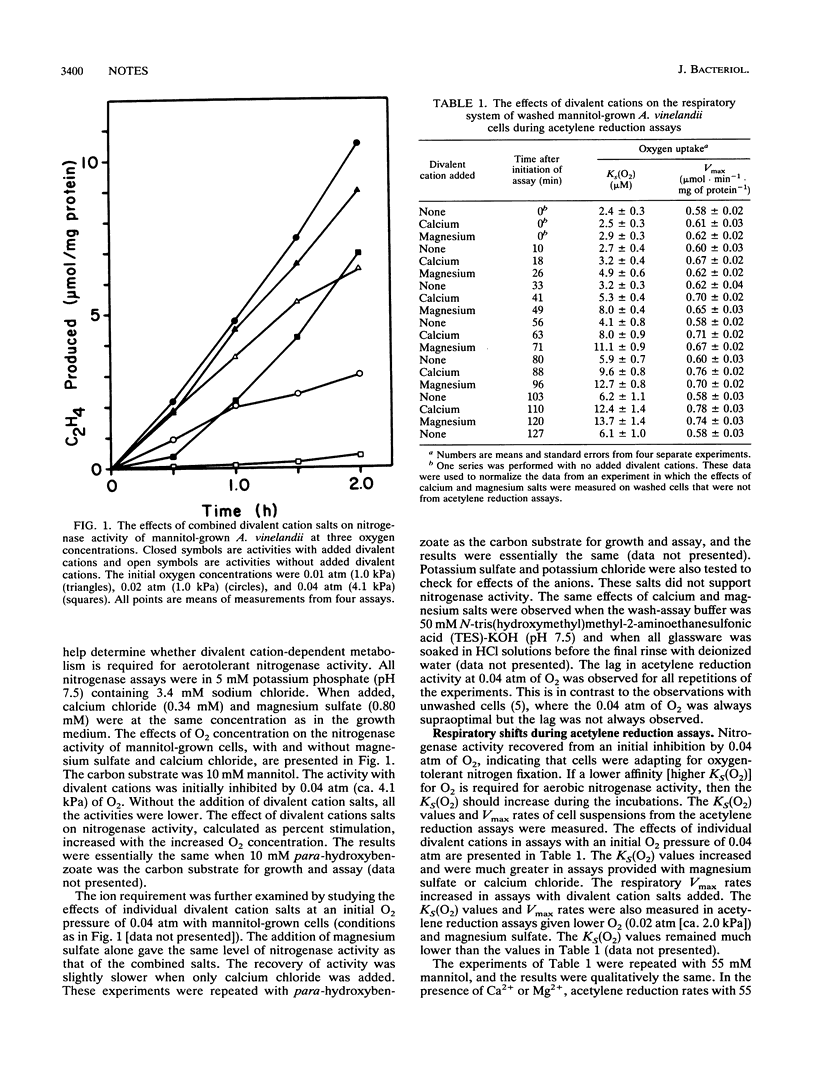
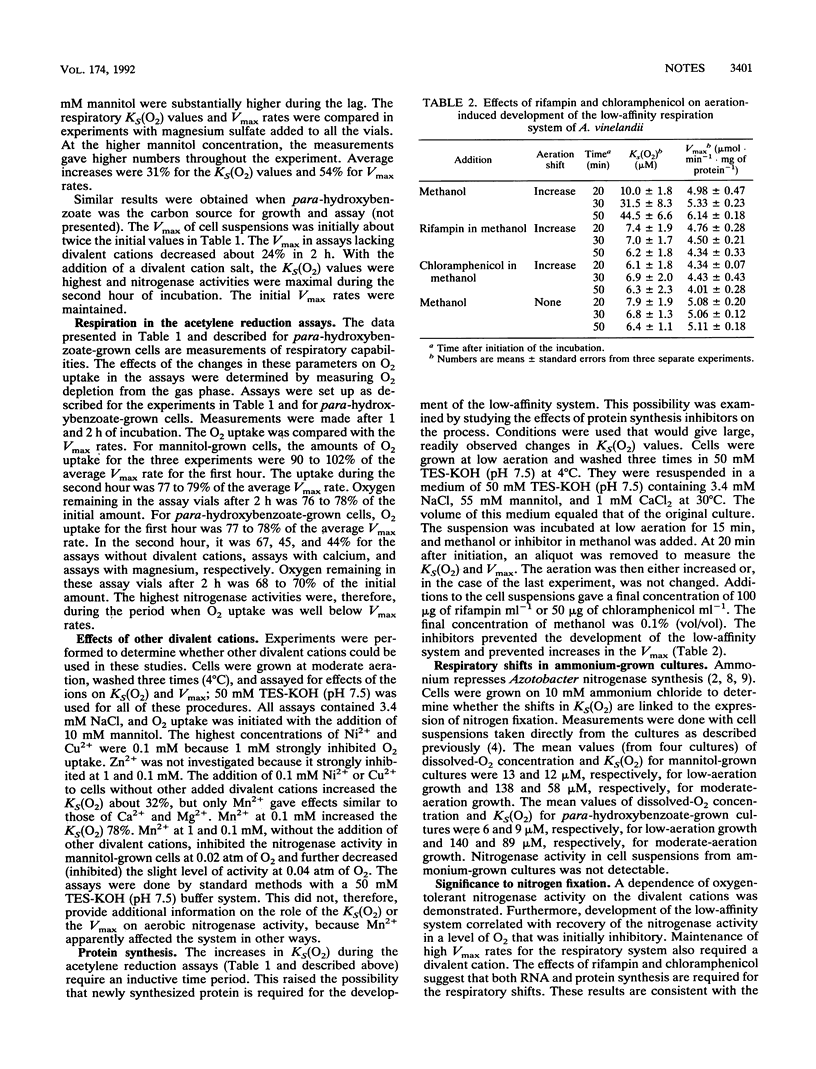
Nitrogenase activity of washed Azotobacter vinelandii cells was enhanced by the addition of Ca2+ and Mg2+, and the enhancement increased with the O2 concentration. In assays provided with a level of O2 that was initially supraoptimal and inhibitory to nitrogenase activity, the addition of Ca2+ or Mg2+ affected both the maximum respiration rate (Vmax) of the cells and the apparent affinity [KS(O2)] of cell respiration for O2. Changes in these parameters correlated with changes in nitrogenase activity. Aeration-dependent increases in Vmax and KS(O2) were inhibited by rifampin and chloramphenicol and were also observed in ammonium-grown cultures.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Krol A. J., Hontelez J. G., Roozendaal B., van Kammen A. On the operon structure of the nitrogenase genes of Rhizobium leguminosarum and Azotobacter vinelandii. Nucleic Acids Res. 1982 Jul 24;10(14):4147–4157. doi: 10.1093/nar/10.14.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Brill W. J. Nitrogenase. I. Repression and derepression of the iron-molybdenum and iron proteins of nitrogenase in Azotobacter vinelandii. Biochim Biophys Acta. 1972 Feb 28;256(2):498–511. doi: 10.1016/0005-2728(72)90078-3. [DOI] [PubMed] [Google Scholar]


