Abstract
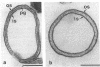
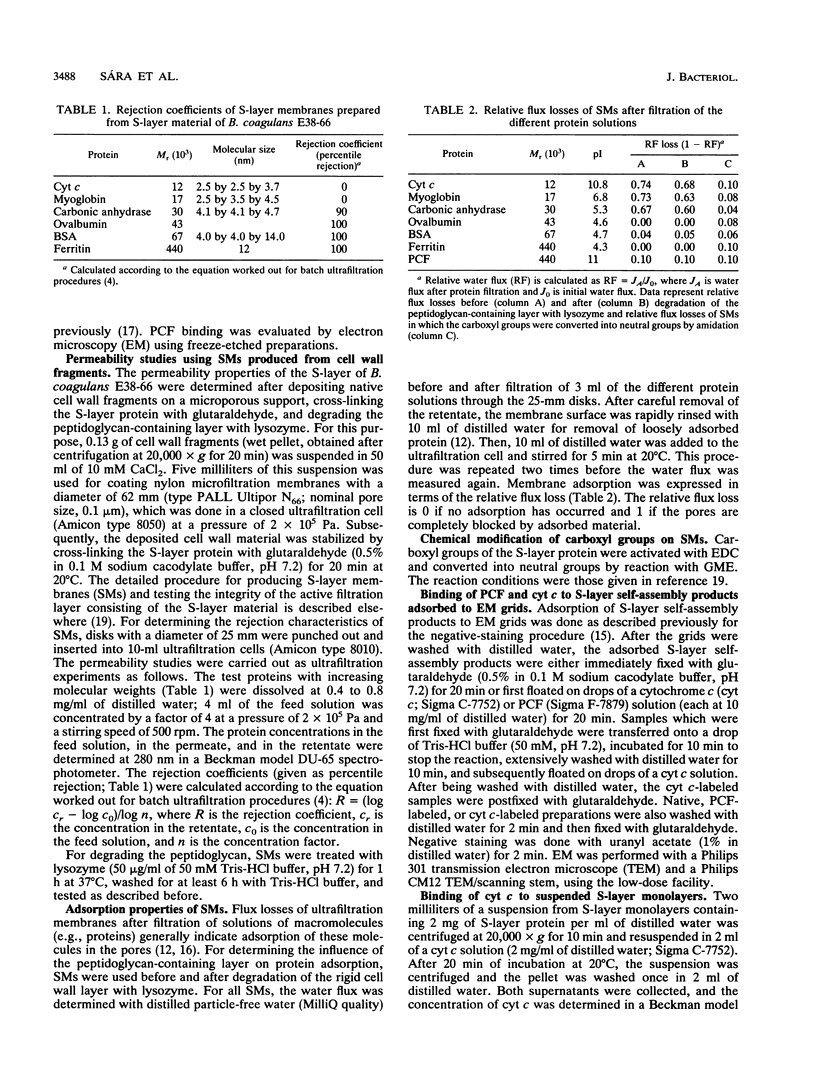
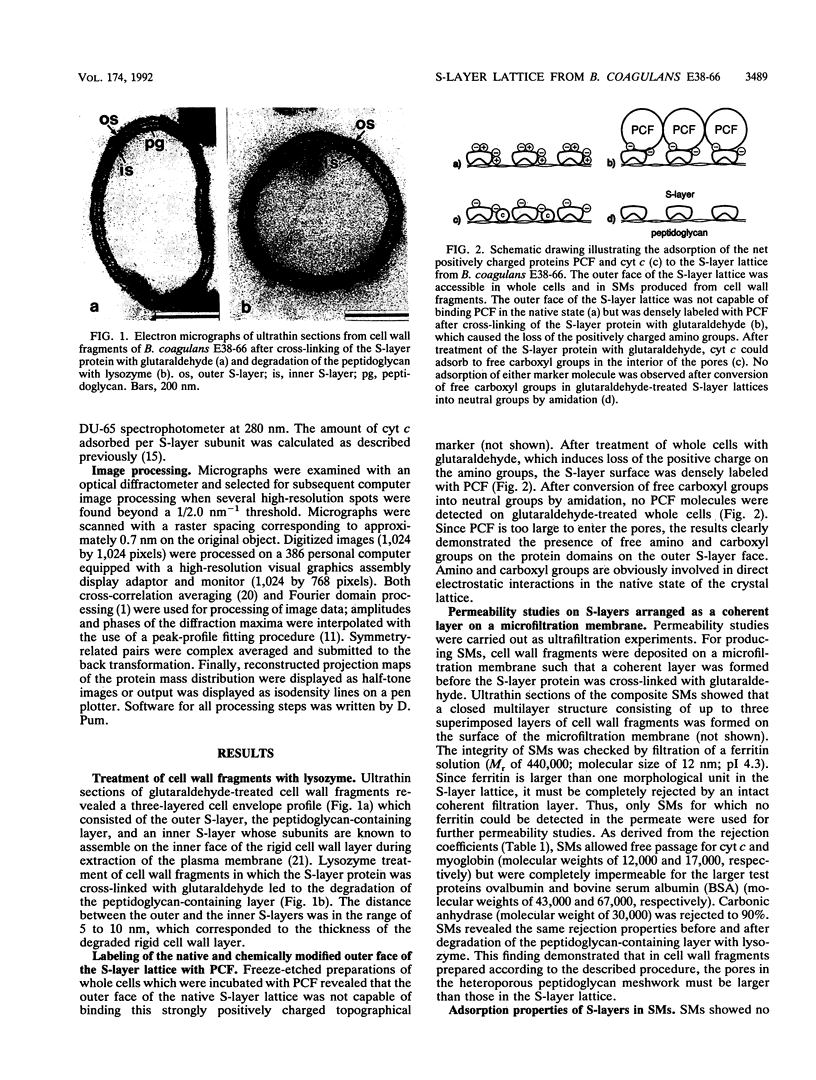
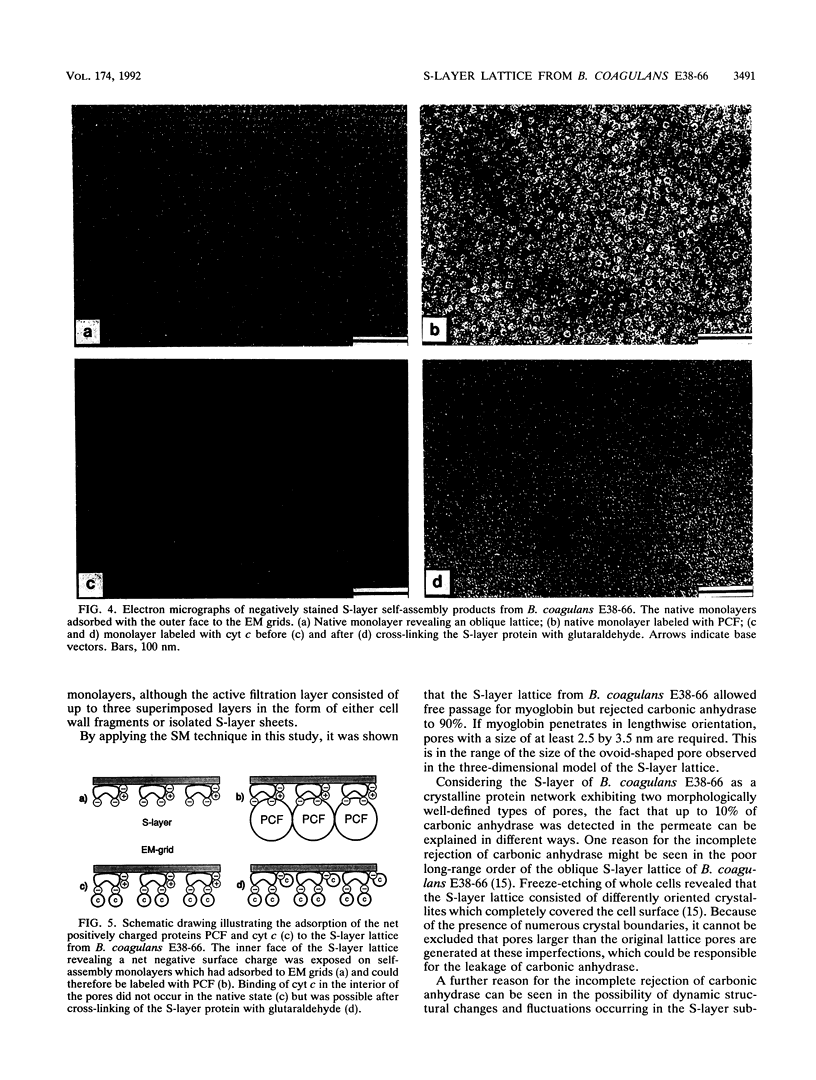
We investigated the permeability properties of the oblique S-layer lattice from Bacillus coagulans E38-66 after depositing cell wall fragments on a microfiltration membrane, cross-linking the S-layer protein with glutaraldehyde, and degrading the peptidoglycan with lysozyme. Comparative permeability studies on such multilayered S-layer membranes and suspended S-layer vesicles from thermophilic members of the family Bacillaceae with use of the space technique (M. Sára and U. B. Sleytr, J. Bacteriol. 169:4092-4098, 1987) revealed identical molecular exclusion limits (M. Sára and U. B. Sleytr, J. Membr. Sci. 33:27-49, 1987). Examination of the S-layer lattice from B. coagulans E38-66 with the S-layer membrane technique revealed unhindered passage for molecules up to the size of myoglobin (M(r) 17,000). The molecular dimensions of this protein (2.8 by 3.2 by 4.5 nm) correspond approximately to the size of the ovoid-shaped pore previously shown by high-resolution electron microscopy of negatively stained S-layer self-assembly products (D. Pum, M. Sára, and U. B. Sleytr, J. Bacteriol. 171:5296-5303, 1989). Chemical modification of the S-layer protein and comparative labeling, adsorption, and permeability studies clearly demonstrated that (i) in the native state, free amino and carboxyl groups are present on the outer S-layer face and in the interior of the pores and (ii) electrostatic interactions between these groups prevent unspecific adsorption of the S-layer in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A., Henderson R., Unwin P. N. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog Biophys Mol Biol. 1982;39(3):183–231. doi: 10.1016/0079-6107(83)90017-2. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Graham L. L. Surface layers of bacteria. Microbiol Rev. 1991 Dec;55(4):684–705. doi: 10.1128/mr.55.4.684-705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- Hovmöller S., Sjögren A., Wang D. N. The structure of crystalline bacterial surface layers. Prog Biophys Mol Biol. 1988;51(2):131–163. doi: 10.1016/0079-6107(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Messner P., Pum D., Sleytr U. B. Characterization of the ultrastructure and the self-assembly of the surface layer of Bacillus stearothermophilus strain NRS 2004/3a. J Ultrastruct Mol Struct Res. 1986 Oct-Dec;97(1-3):73–88. doi: 10.1016/s0889-1605(86)80008-8. [DOI] [PubMed] [Google Scholar]
- Messner P., Sleytr U. B. Crystalline bacterial cell-surface layers. Adv Microb Physiol. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W., Hahn M. Three-dimensional reconstruction of imperfect two-dimensional crystals. Ultramicroscopy. 1984;13(1-2):57–70. doi: 10.1016/0304-3991(84)90057-3. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988 Jul;170(7):2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sára M., Sleytr U. B. Charge distribution on the S layer of Bacillus stearothermophilus NRS 1536/3c and importance of charged groups for morphogenesis and function. J Bacteriol. 1987 Jun;169(6):2804–2809. doi: 10.1128/jb.169.6.2804-2809.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sára M., Sleytr U. B. Molecular sieving through S layers of Bacillus stearothermophilus strains. J Bacteriol. 1987 Sep;169(9):4092–4098. doi: 10.1128/jb.169.9.4092-4098.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]