Abstract
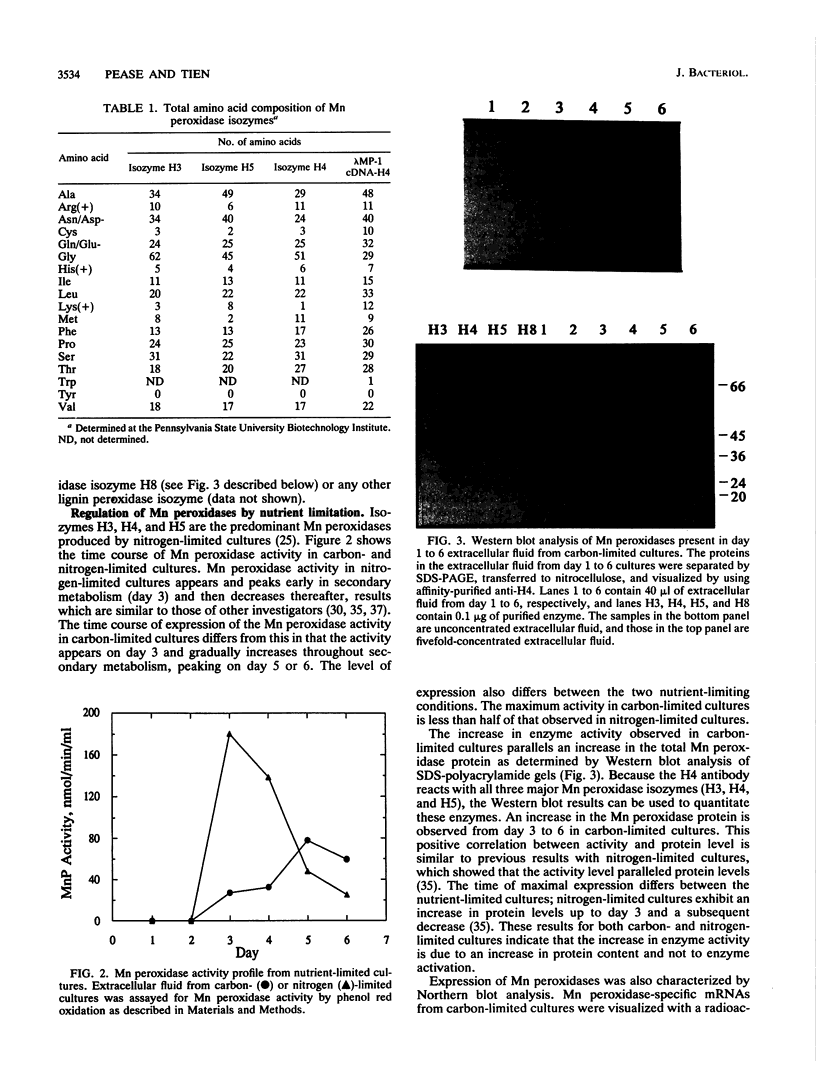
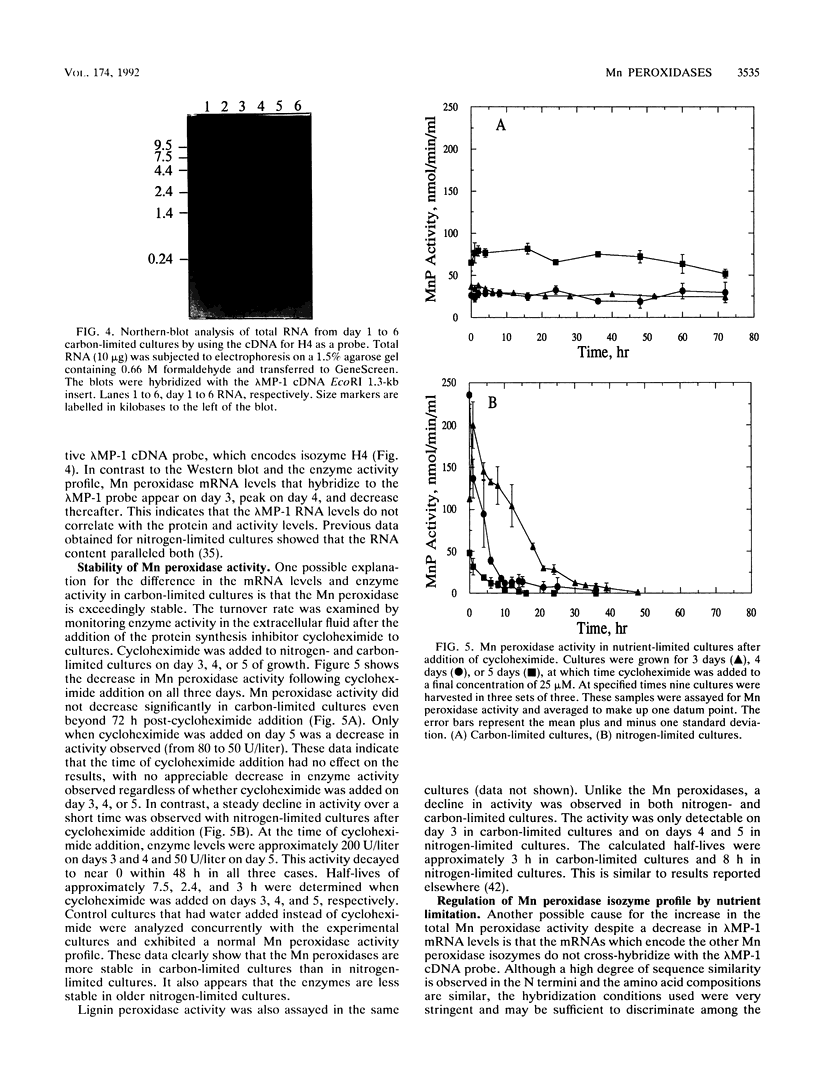
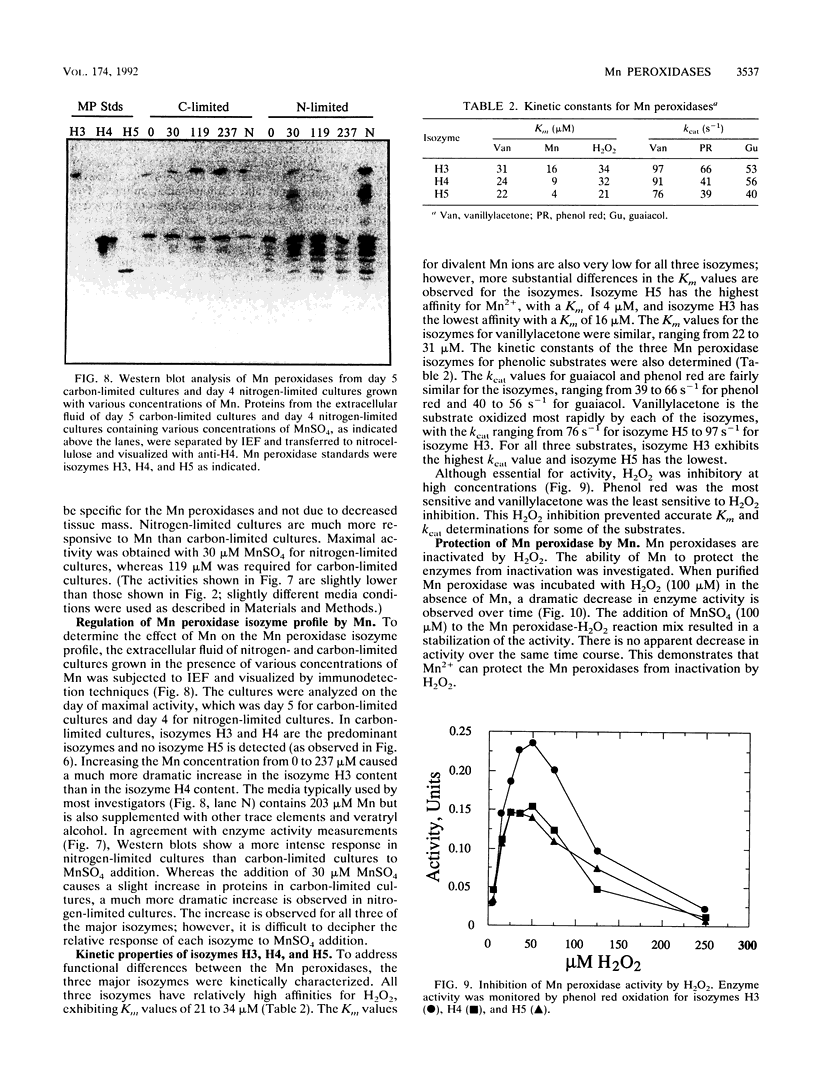
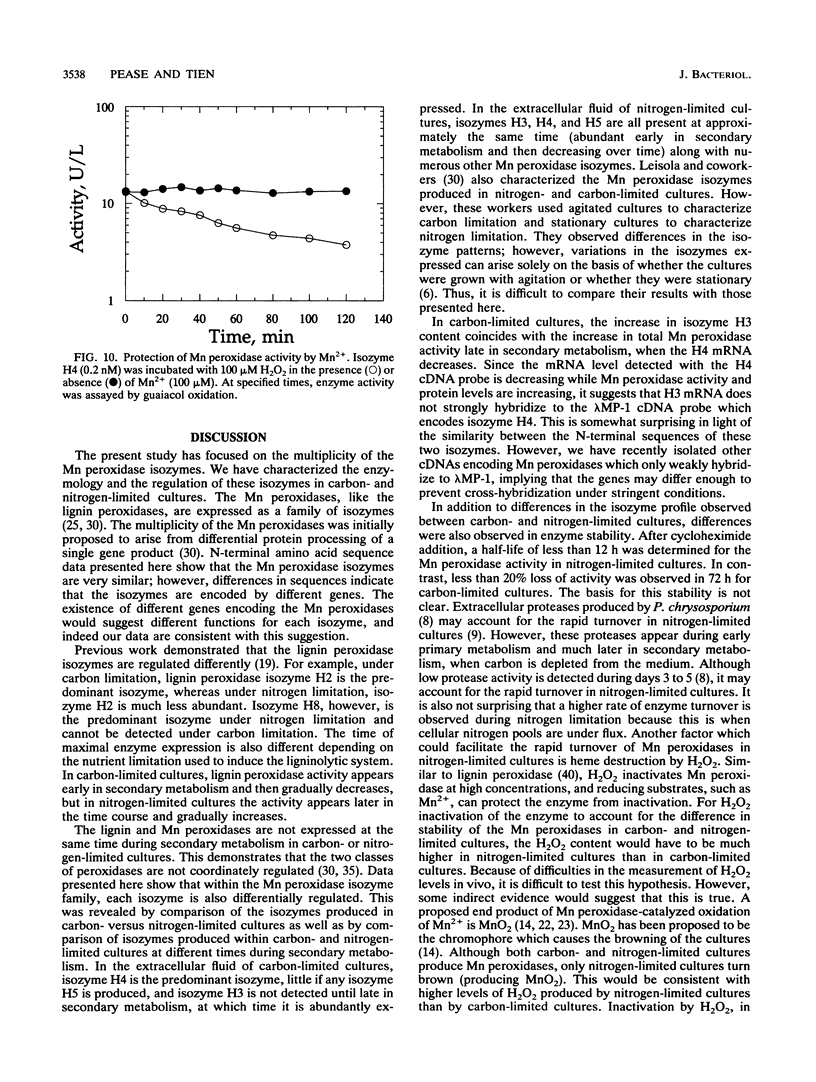
Lignin and Mn peroxidases are two families of isozymes produced by the lignin-degrading fungus Phanerochaete chrysosporium under nutrient nitrogen or carbon limitation. We purified to homogeneity the three major Mn peroxidase isozymes, H3 (pI = 4.9), H4 (pI = 4.5), and H5 (pI = 4.2). Amino-terminal sequencing of these isozymes demonstrates that they are encoded by different genes. We also analyzed the regulation of these isozymes in carbon- and nitrogen-limited cultures and found not only that the lignin and Mn peroxidases are differentially regulated but also that differential regulation occurs within the Mn peroxidase isozyme family. The isozyme profile and the time at which each isozyme appears in secondary metabolism differ in both nitrogen- and carbon-limited cultures. Each isozyme also responded differently to the addition of a putative inducer, divalent Mn. The stability of the Mn peroxidases in carbon- and nitrogen-limited cultures was also characterized after cycloheximide addition. The Mn peroxidases are more stable in carbon-limited cultures than in nitrogen-limited cultures. They are also more stable than the lignin peroxidases. These data collectively suggest that the Mn peroxidase isozymes serve different functions in lignin biodegradation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. A., Glenn J. K., Gold M. H. Manganese regulates expression of manganese peroxidase by Phanerochaete chrysosporium. J Bacteriol. 1990 Jun;172(6):3125–3130. doi: 10.1128/jb.172.6.3125-3130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass S. B., Reddy C. A. Characterization of extracellular peroxidases produced by acetate-buffered cultures of the lignin-degrading basidiomycete Phanerochaete chrysosporium. FEMS Microbiol Lett. 1990 Jun 1;57(3):221–224. doi: 10.1111/j.1574-6968.1990.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Datta A., Bettermann A., Kirk T. K. Identification of a specific manganese peroxidase among ligninolytic enzymes secreted by Phanerochaete chrysosporium during wood decay. Appl Environ Microbiol. 1991 May;57(5):1453–1460. doi: 10.1128/aem.57.5.1453-1460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoretz C. G., Chen H. C., Grethlein H. E. Effect of Environmental Conditions on Extracellular Protease Activity in Lignolytic Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Feb;56(2):395–400. doi: 10.1128/aem.56.2.395-400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoretz C. G., Dass S. B., Reddy C. A., Grethlein H. E. Protease-mediated degradation of lignin peroxidase in liquid cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Nov;56(11):3429–3434. doi: 10.1128/aem.56.11.3429-3434.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Factors Involved in the Regulation of a Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Feb;49(2):299–304. doi: 10.1128/aem.49.2.299-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Tien M., Aust S. D. The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungus Phanerochaete chrysosporium. J Biol Chem. 1982 Oct 10;257(19):11455–11462. [PubMed] [Google Scholar]
- Glenn J. K., Akileswaran L., Gold M. H. Mn(II) oxidation is the principal function of the extracellular Mn-peroxidase from Phanerochaete chrysosporium. Arch Biochem Biophys. 1986 Dec;251(2):688–696. doi: 10.1016/0003-9861(86)90378-4. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Holzbaur E. L., Andrawis A., Tien M. Structure and regulation of a lignin peroxidase gene from Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1988 Sep 15;155(2):626–633. doi: 10.1016/s0006-291x(88)80541-2. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern D. H., Weisenthal L. M. Highly specific prediction of antineoplastic drug resistance with an in vitro assay using suprapharmacologic drug exposures. J Natl Cancer Inst. 1990 Apr 4;82(7):582–588. doi: 10.1093/jnci/82.7.582. [DOI] [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Tien M., Kersten P. J., Mozuch M. D., Kalyanaraman B. Ligninase of Phanerochaete chrysosporium. Mechanism of its degradation of the non-phenolic arylglycerol beta-aryl ether substructure of lignin. Biochem J. 1986 May 15;236(1):279–287. doi: 10.1042/bj2360279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leisola M. S., Kozulic B., Meussdoerffer F., Fiechter A. Homology among multiple extracellular peroxidases from Phanerochaete chrysosporium. J Biol Chem. 1987 Jan 5;262(1):419–424. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- Orth A. B., Denny M., Tien M. Overproduction of lignin-degrading enzymes by an isolate of Phanerochaete chrysosporium. Appl Environ Microbiol. 1991 Sep;57(9):2591–2596. doi: 10.1128/aem.57.9.2591-2596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszczyński A., Huynh V. B., Crawford R. Comparison of ligninase-I and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. Arch Biochem Biophys. 1986 Feb 1;244(2):750–765. doi: 10.1016/0003-9861(86)90644-2. [DOI] [PubMed] [Google Scholar]
- Pease E. A., Andrawis A., Tien M. Manganese-dependent peroxidase from Phanerochaete chrysosporium. Primary structure deduced from cDNA sequence. J Biol Chem. 1989 Aug 15;264(23):13531–13535. [PubMed] [Google Scholar]
- Pease E. A., Aust S. D., Tien M. Heterologous expression of active manganese peroxidase from Phanerochaete chrysosporium using the baculovirus expression system. Biochem Biophys Res Commun. 1991 Sep 16;179(2):897–903. doi: 10.1016/0006-291x(91)91903-p. [DOI] [PubMed] [Google Scholar]
- Pribnow D., Mayfield M. B., Nipper V. J., Brown J. A., Gold M. H. Characterization of a cDNA encoding a manganese peroxidase, from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Biol Chem. 1989 Mar 25;264(9):5036–5040. [PubMed] [Google Scholar]
- Tien M., Kirk T. K., Bull C., Fee J. A. Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by the ligninase of Phanerocheate chrysosporium Burds. J Biol Chem. 1986 Feb 5;261(4):1687–1693. [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Myer S. B. Selection and characterization of mutants of Phanerochaete chrysosporium exhibiting ligninolytic activity under nutrient-rich conditions. Appl Environ Microbiol. 1990 Aug;56(8):2540–2544. doi: 10.1128/aem.56.8.2540-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonon F., Odier E. Influence of Veratryl Alcohol and Hydrogen Peroxide on Ligninase Activity and Ligninase Production by Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 Feb;54(2):466–472. doi: 10.1128/aem.54.2.466-472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]






