Abstract
The DNA sequence of the dnaK gene of Escherichia coli was analyzed. The nucleotide sequence of the wild-type dnaK gene of E. coli B differed from that of E. coli K-12 in 15 bp, none of which altered the amino acid sequence. Two temperature-sensitive dnaK mutations were examined by cloning and sequence analyses. Results showed that one dnaK mutation, dnaK7(Ts), was a one-base substitution of T for C at nucleotide position 448 in the open reading frame yielding an amber nonsense codon. The other mutation, dnaK756(Ts), consisted of base substitutions (A for G) at three nucleotide positions, 95, 1364, and 1403, in the open reading frame resulting in an aspartic acid codon in place of a glycine codon.
Full text
PDF


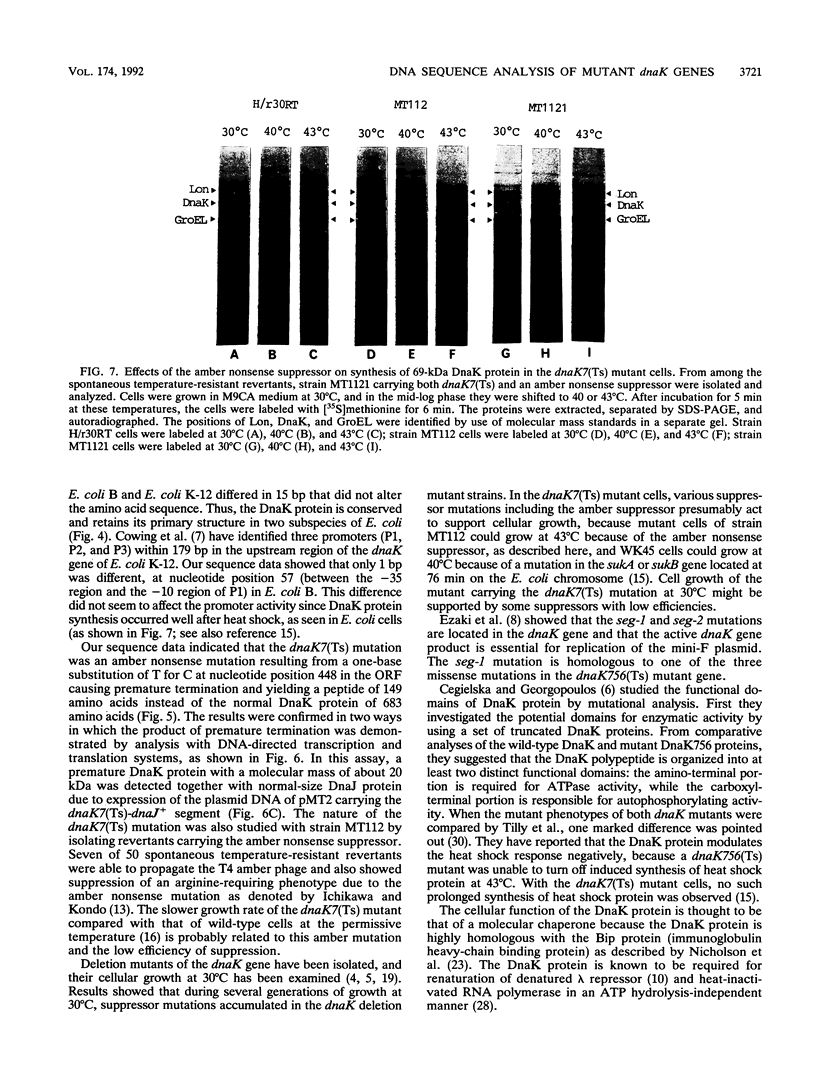

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Tilly K., Craig E., King J., Zylicz M., Georgopoulos C. The nucleotide sequence of the Escherichia coli K12 dnaJ+ gene. A gene that encodes a heat shock protein. J Biol Chem. 1986 Feb 5;261(4):1782–1785. [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bacteriol. 1989 May;171(5):2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Mutations altering heat shock specific subunit of RNA polymerase suppress major cellular defects of E. coli mutants lacking the DnaK chaperone. EMBO J. 1990 Dec;9(12):4027–4036. doi: 10.1002/j.1460-2075.1990.tb07624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegielska A., Georgopoulos C. Functional domains of the Escherichia coli dnaK heat shock protein as revealed by mutational analysis. J Biol Chem. 1989 Dec 15;264(35):21122–21130. [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki B., Ogura T., Mori H., Niki H., Hiraga S. Involvement of DnaK protein in mini-F plasmid replication: temperature-sensitive seg mutations are located in the dnaK gene. Mol Gen Genet. 1989 Aug;218(2):183–189. doi: 10.1007/BF00331267. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gaitanaris G. A., Papavassiliou A. G., Rubock P., Silverstein S. J., Gottesman M. E. Renaturation of denatured lambda repressor requires heat shock proteins. Cell. 1990 Jun 15;61(6):1013–1020. doi: 10.1016/0092-8674(90)90066-n. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. A new bacterial gene (groPC) which affects lambda DNA replication. Mol Gen Genet. 1977 Feb 28;151(1):35–39. doi: 10.1007/BF00446910. [DOI] [PubMed] [Google Scholar]
- Itikawa H., Fujita H., Wada M. High temperature induction of a stringent response in the dnaK(Ts) and dnaJ(Ts) mutants of Escherichia coli. J Biochem. 1986 Jun;99(6):1719–1724. doi: 10.1093/oxfordjournals.jbchem.a135648. [DOI] [PubMed] [Google Scholar]
- Itikawa H., Mishina Y., Wada M., Fujita H. Genetic mapping and biochemical characterization of suppressor mutations sukA and sukB for a dnaK7(Ts) mutation of Escherichia coli K-12. Jpn J Genet. 1992 Feb;67(1):17–27. doi: 10.1266/jjg.67.17. [DOI] [PubMed] [Google Scholar]
- Itikawa H., Ryu J. Isolation and characterization of a temperature-sensitive dnaK mutant of Escherichia coli B. J Bacteriol. 1979 May;138(2):339–344. doi: 10.1128/jb.138.2.339-344.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itikawa H., Wada M., Sekine K., Fujita H. Phosphorylation of glutaminyl-tRNA synthetase and threonyl-tRNA synthetase by the gene products of dnaK and dnaJ in Escherichia coli K-12 cells. Biochimie. 1989 Sep-Oct;71(9-10):1079–1087. doi: 10.1016/0300-9084(89)90114-4. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Shiba T., Nakata A., Shinagawa H. Involvement in DNA repair of the ruvA gene of Escherichia coli. Mol Gen Genet. 1989 Oct;219(1-2):328–331. doi: 10.1007/BF00261196. [DOI] [PubMed] [Google Scholar]
- Kang P. J., Craig E. A. Identification and characterization of a new Escherichia coli gene that is a dosage-dependent suppressor of a dnaK deletion mutation. J Bacteriol. 1990 Apr;172(4):2055–2064. doi: 10.1128/jb.172.4.2055-2064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease III of Haemophilus influenzae and restriction endonuclease I of Escherichia coli. J Mol Biol. 1975 Nov 5;98(3):551–564. doi: 10.1016/s0022-2836(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Nicholson R. C., Williams D. B., Moran L. A. An essential member of the HSP70 gene family of Saccharomyces cerevisiae is homologous to immunoglobulin heavy chain binding protein. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1159–1163. doi: 10.1073/pnas.87.3.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki M., Tamura F., Nishimura S., Uchida H. Nucleotide sequence of the Escherichia coli dnaJ gene and purification of the gene product. J Biol Chem. 1986 Feb 5;261(4):1778–1781. [PubMed] [Google Scholar]
- Saito H., Uchida H. Initiation of the DNA replication of bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1977 Jun 15;113(1):1–25. doi: 10.1016/0022-2836(77)90038-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D., Georgopoulos C., Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990 Sep 7;62(5):939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- Sunshine M., Feiss M., Stuart J., Yochem J. A new host gene (groPC) necessary for lambda DNA replication. Mol Gen Genet. 1977 Feb 28;151(1):27–34. doi: 10.1007/BF00446909. [DOI] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wada M., Fujita H., Itikawa H. Genetic suppression of a temperature-sensitive groES mutation by an altered subunit of RNA polymerase of Escherichia coli K-12. J Bacteriol. 1987 Mar;169(3):1102–1106. doi: 10.1128/jb.169.3.1102-1106.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Itikawa H. Participation of Escherichia coli K-12 groE gene products in the synthesis of cellular DNA and RNA. J Bacteriol. 1984 Feb;157(2):694–696. doi: 10.1128/jb.157.2.694-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Sekine K., Itikawa H. Participation of the dnaK and dnaJ gene products in phosphorylation of glutaminyl-tRNA synthetase and threonyl-tRNA synthetase of Escherichia coli K-12. J Bacteriol. 1986 Oct;168(1):213–220. doi: 10.1128/jb.168.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yochem J., Uchida H., Sunshine M., Saito H., Georgopoulos C. P., Feiss M. Genetic analysis of two genes, dnaJ and dnaK, necessary for Escherichia coli and bacteriophage lambda DNA replication. Mol Gen Genet. 1978 Aug 4;164(1):9–14. doi: 10.1007/BF00267593. [DOI] [PubMed] [Google Scholar]
- Zylicz M., LeBowitz J. H., McMacken R., Georgopoulos C. The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]