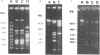Abstract
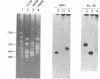
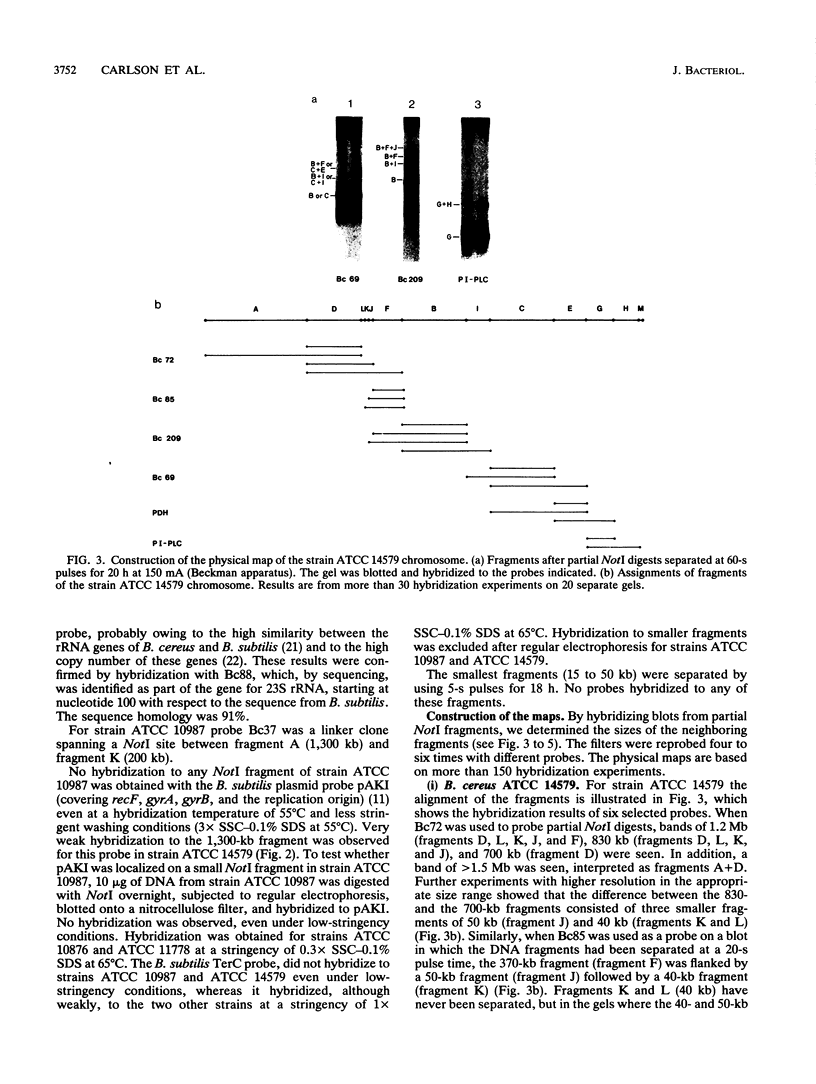
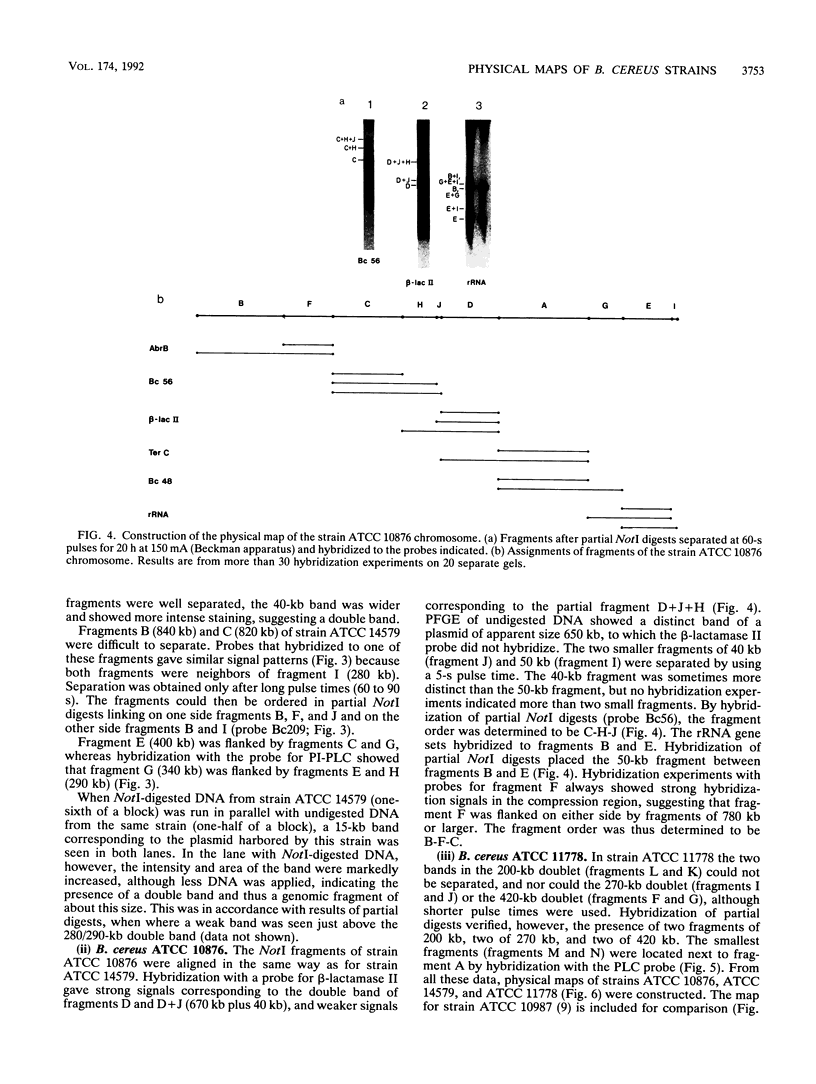
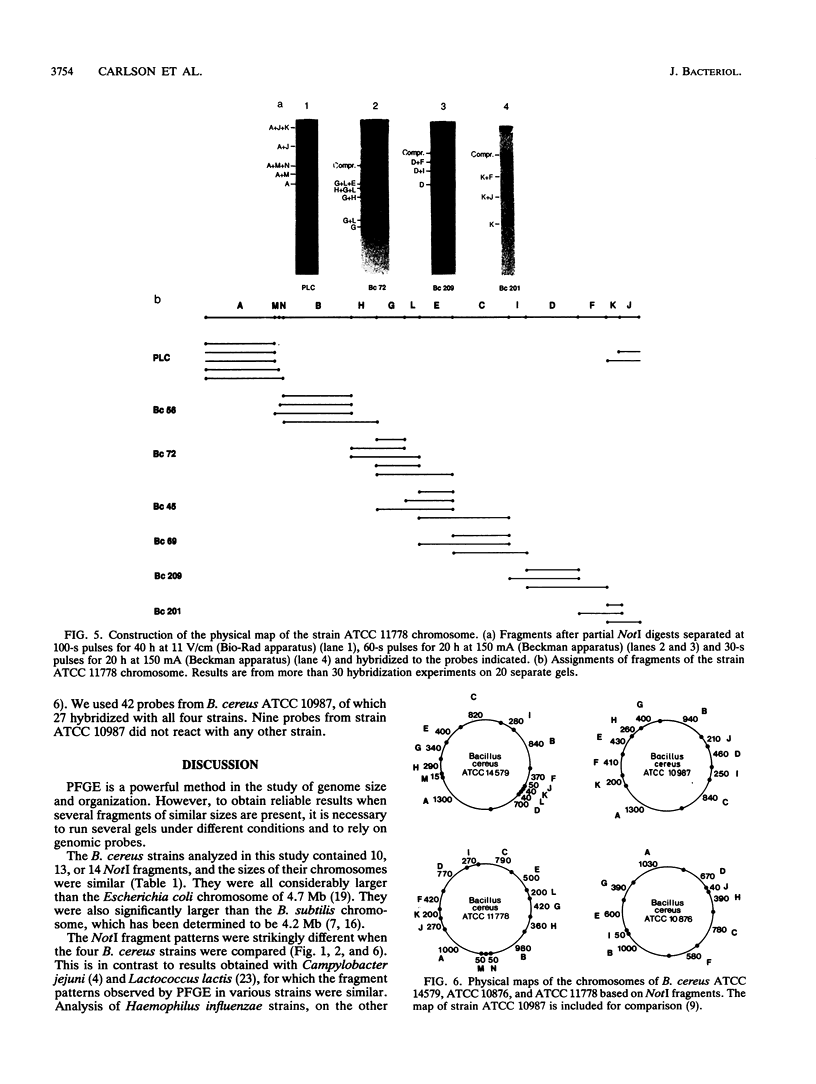
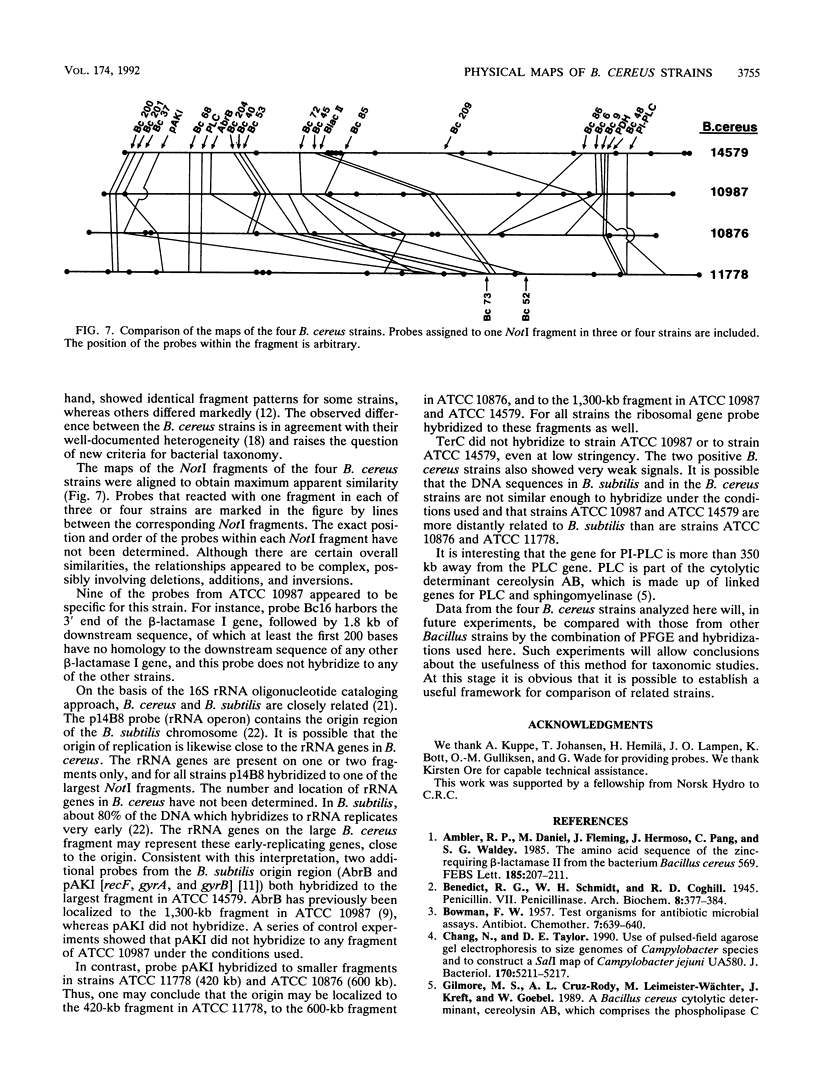
NotI restriction maps of the chromosomes from Bacillus cereus ATCC 10876, ATCC 11778, and the B. cereus type strain ATCC 14579 have been established and compared with the previously established map of B. cereus ATCC 10987. Between 10 and 14 NotI fragments were observed, ranging from 15 to 1,300 kb, in digests of DNA from the various strains. The sizes of the genomes varied between 5.4 and 6.3 Mb. The maps were constructed by hybridization of 42 random probes, prepared from B. cereus ATCC 10987 libraries, to fragments from partial and complete NotI digests, separated by pulsed-field gel electrophoresis. Nine probes were specific for ATCC 10987 only. Probes for five B. subtilis and five B. cereus genes were also used. The NotI restriction fragment patterns of the four strains were strikingly different.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Daniel M., Fleming J., Hermoso J. M., Pang C., Waley S. G. The amino acid sequence of the zinc-requiring beta-lactamase II from the bacterium Bacillus cereus 569. FEBS Lett. 1985 Sep 23;189(2):207–211. doi: 10.1016/0014-5793(85)81024-3. [DOI] [PubMed] [Google Scholar]
- Chang N., Taylor D. E. Use of pulsed-field agarose gel electrophoresis to size genomes of Campylobacter species and to construct a SalI map of Campylobacter jejuni UA580. J Bacteriol. 1990 Sep;172(9):5211–5217. doi: 10.1128/jb.172.9.5211-5217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya M., Tanaka T. Complete physical map of the Bacillus subtilis 168 chromosome constructed by a gene-directed mutagenesis method. J Mol Biol. 1991 Aug 5;220(3):631–648. doi: 10.1016/0022-2836(91)90106-g. [DOI] [PubMed] [Google Scholar]
- Johansen T., Holm T., Guddal P. H., Sletten K., Haugli F. B., Little C. Cloning and sequencing of the gene encoding the phosphatidylcholine-preferring phospholipase C of Bacillus cereus. Gene. 1988 May 30;65(2):293–304. doi: 10.1016/0378-1119(88)90466-0. [DOI] [PubMed] [Google Scholar]
- Kolstø A. B., Grønstad A., Oppegaard H. Physical map of the Bacillus cereus chromosome. J Bacteriol. 1990 Jul;172(7):3821–3825. doi: 10.1128/jb.172.7.3821-3825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppe A., Evans L. M., McMillen D. A., Griffith O. H. Phosphatidylinositol-specific phospholipase C of Bacillus cereus: cloning, sequencing, and relationship to other phospholipases. J Bacteriol. 1989 Nov;171(11):6077–6083. doi: 10.1128/jb.171.11.6077-6083.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M. F., Bott K. F. Genetic and physical organization of the cloned gyrA and gyrB genes of Bacillus subtilis. J Bacteriol. 1985 Apr;162(1):78–84. doi: 10.1128/jb.162.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Smith H. O., Redfield R. J. Organization of the Haemophilus influenzae Rd genome. J Bacteriol. 1989 Jun;171(6):3016–3024. doi: 10.1128/jb.171.6.3016-3024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S. Translational attenuation as the regulator of inducible cat genes. J Bacteriol. 1990 Jan;172(1):1–6. doi: 10.1128/jb.172.1.1-6.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna M. J., Zhu Y. F., Lampen J. O. Nucleotide sequence of the beta-lactamase I gene of Bacillus cereus strains 569/H and 5/B. Nucleic Acids Res. 1987 Feb 25;15(4):1877–1877. doi: 10.1093/nar/15.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Beta-lactamase III of Bacillus cereus 569: membrane lipoprotein and secreted protein. Biochemistry. 1983 Sep 27;22(20):4652–4656. doi: 10.1021/bi00289a007. [DOI] [PubMed] [Google Scholar]
- Otnaess A. B., Little C., Sletten K., Wallin R., Johnsen S., Flengsrud R., Prydz H. Some characteristics of phospholipase C from Bacillus cereus. Eur J Biochem. 1977 Oct 3;79(2):459–468. doi: 10.1111/j.1432-1033.1977.tb11828.x. [DOI] [PubMed] [Google Scholar]
- Priest F. G., Goodfellow M., Todd C. A numerical classification of the genus Bacillus. J Gen Microbiol. 1988 Jul;134(7):1847–1882. doi: 10.1099/00221287-134-7-1847. [DOI] [PubMed] [Google Scholar]
- SMITH N. R., GIBSON T., GORDON R. E., SNEATH P. H. TYPE CULTURES AND PROPOSED NEOTYPE CULTURES OF SOME SPECIES IN THE GENUS BACILLUS. J Gen Microbiol. 1964 Feb;34:269–272. doi: 10.1099/00221287-34-2-269. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E., Ludwig W., Weizenegger M., Dorn S., McGill T. J., Fox G. E., Woese C. R., Schubert W., Schleifer K. H. Comparative 16S rRNA oligonucleotide analyses and murein types of round-spore-forming bacilli and non-spore-forming relatives. J Gen Microbiol. 1987 Sep;133(9):2523–2529. doi: 10.1099/00221287-133-9-2523. [DOI] [PubMed] [Google Scholar]
- Stewart G. C., Wilson F. E., Bott K. F. Detailed physical mapping of the ribosomal RNA genes of Bacillus subtilis. Gene. 1982 Sep;19(2):153–162. doi: 10.1016/0378-1119(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Tanskanen E. I., Tulloch D. L., Hillier A. J., Davidson B. E. Pulsed-Field Gel Electrophoresis of SmaI Digests of Lactococcal Genomic DNA, a Novel Method of Strain Identification. Appl Environ Microbiol. 1990 Oct;56(10):3105–3111. doi: 10.1128/aem.56.10.3105-3111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]