Abstract
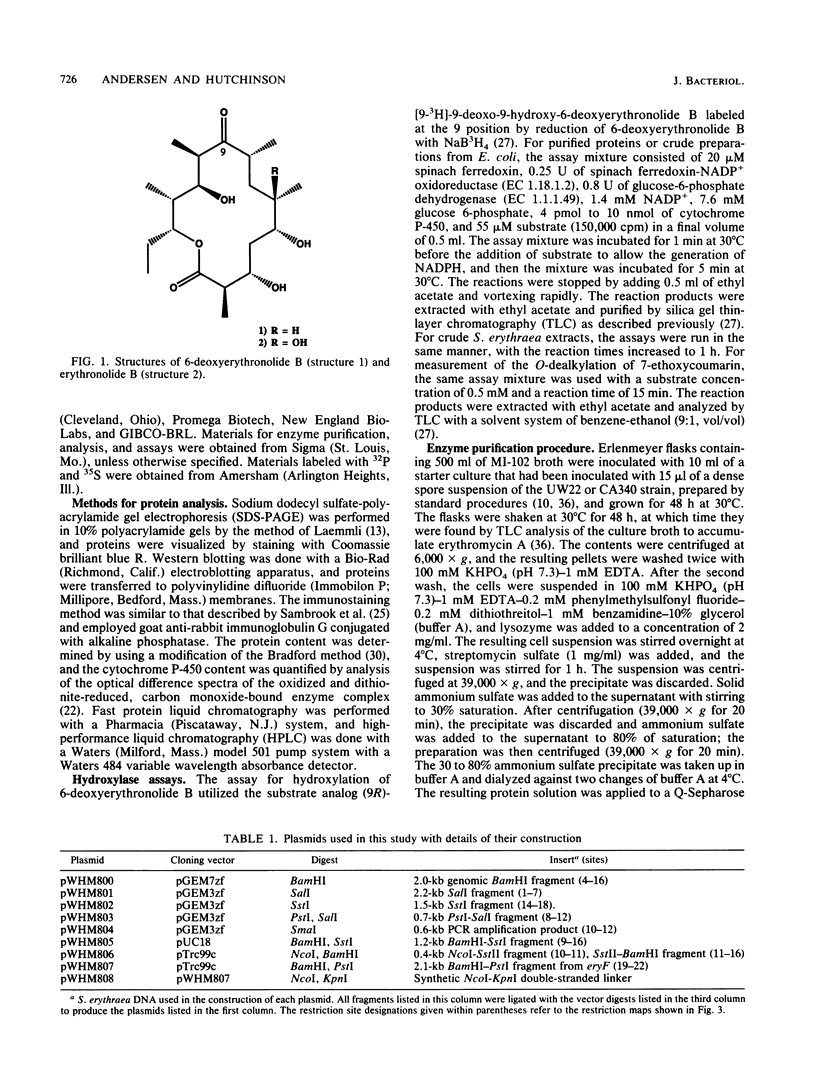
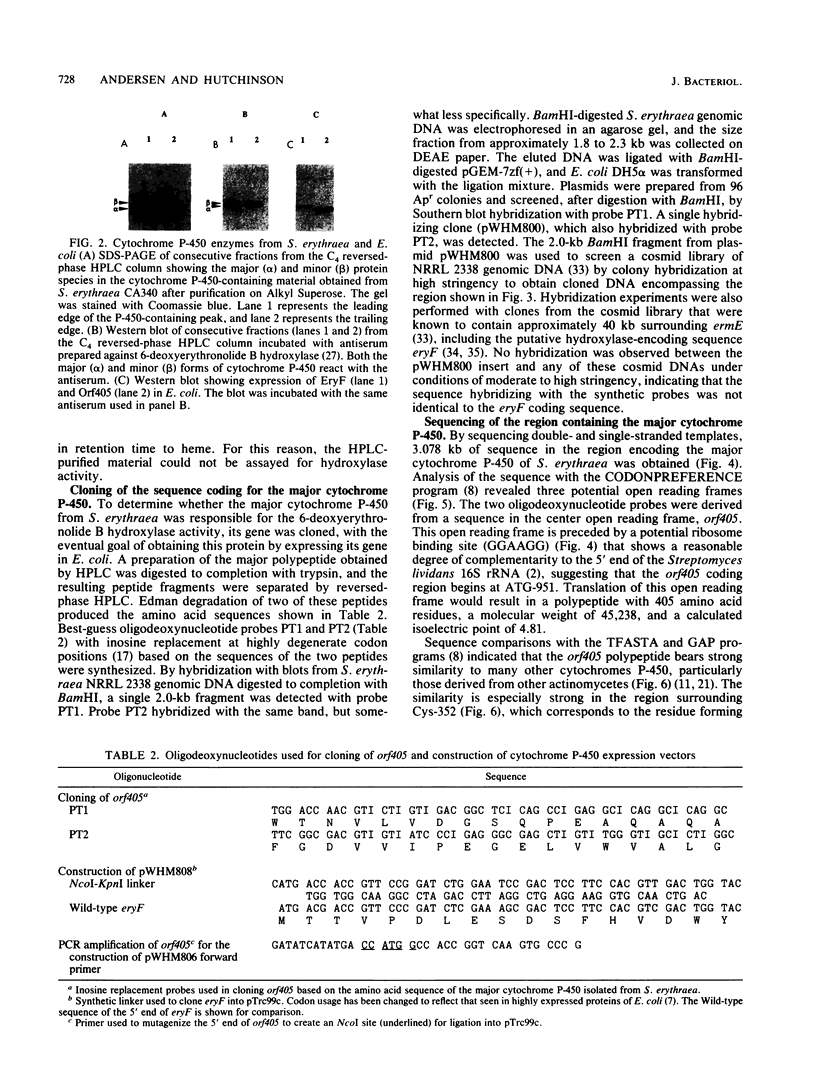
Previous studies of erythromycin biosynthesis have indicated that a cytochrome P-450 monooxygenase system is responsible for hydroxylation of 6-deoxyerythronolide B to erythronolide B as part of erythromycin biosynthesis in Saccharopolyspora erythraea (A. Shafiee and C. R. Hutchinson, Biochemistry 26:6204-6210 1987). The enzyme was previously purified to apparent homogeneity and found to have a catalytic turnover number of approximately 10(-3) min-1. More recently, disruption of a P-450-encoding sequence (eryF) in the region of ermE, the erythromycin resistance gene of S. erythraea, produced a 6-deoxyerythronolide B hydroxylation-deficient mutant (J. M. Weber, J. O. Leung, S. J. Swanson, K. B. Idler, and J. B. McAlpine, Science 252:114-116, 1991). In this study we purified the catalytically active cytochrome P-450 fraction from S. erythraea and found by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis that it consists of a major and a minor P-450 species. The gene encoding the major species (orf405) was cloned from genomic DNA and found to be distinct from eryF. Both the orf405 and eryF genes were expressed in Escherichia coli, and the properties of the proteins were compared. Heterologously expressed EryF and Orf405 both reacted with antisera prepared against the 6-deoxyerythronolide B hydroxylase described by Shafiee and Hutchinson (1987), and the EryF polypeptide comigrated with the minor P-450 species from S. erythraea on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. In comparisons of enzymatic activity, EryF hydroxylated a substrate with a turnover number of 53 min-1, whereas Orf405 showed no detectable activity with a 6-deoxyerythronolide B analog. Both enzymes showed weak activity in the O-dealkylation of 7-ethoxycoumarin. We conclude that the previously isolated 6-deoxyerythronolide B hydroxylase was a mixture of two P-450 enzymes and that only the minor form shows 6-deoxyerythronolide B hydroxylase activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Ochs B., Abel K. J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988 Sep 30;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Cohen S. N. Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet. 1982;187(2):265–277. doi: 10.1007/BF00331128. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Corcoran J. W. S-adenosylmethionine:erythromycin C O-methyltransferase. Methods Enzymol. 1975;43:487–498. doi: 10.1016/0076-6879(75)43109-3. [DOI] [PubMed] [Google Scholar]
- Corcoran J. W., Vygantas A. M. Accumulation of 6-deoxyerythronolide B in a normal strain of Streptomyces erythreus and hydroxylation at carbon 6 of the erythranolide ring system by a soluble noninduced cell-free enzyme system. Biochemistry. 1982 Jan 19;21(2):263–269. doi: 10.1021/bi00531a010. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
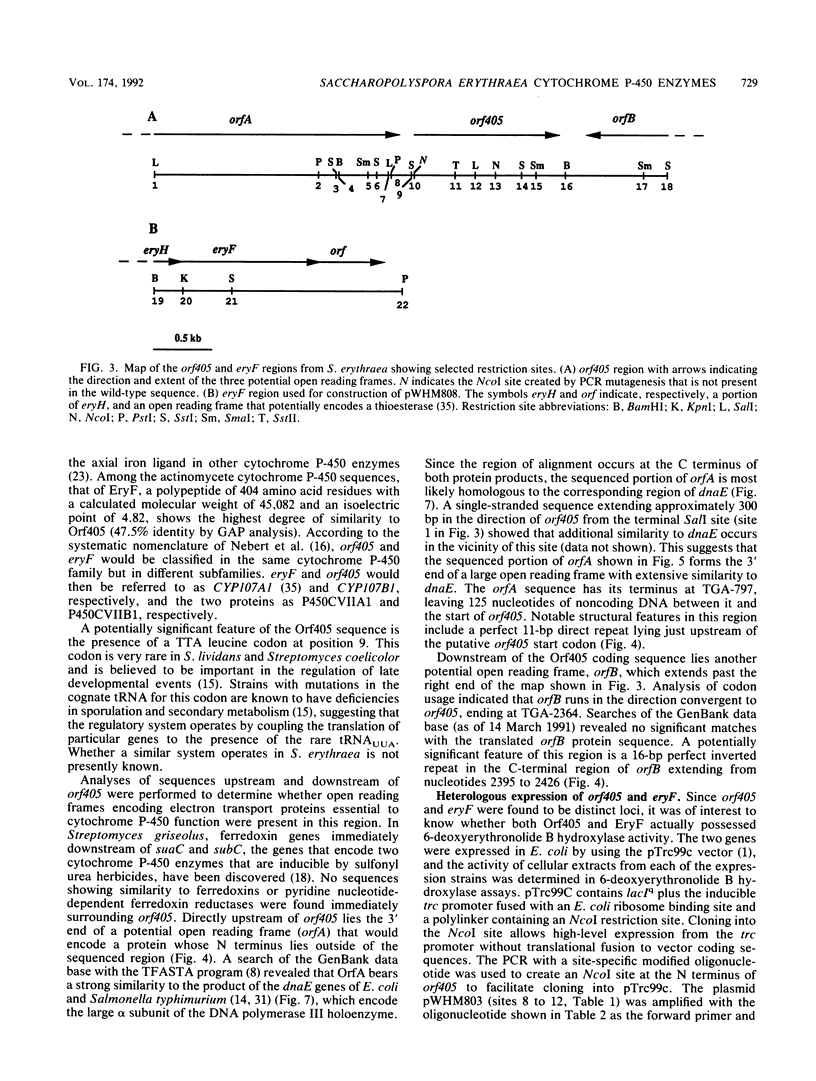
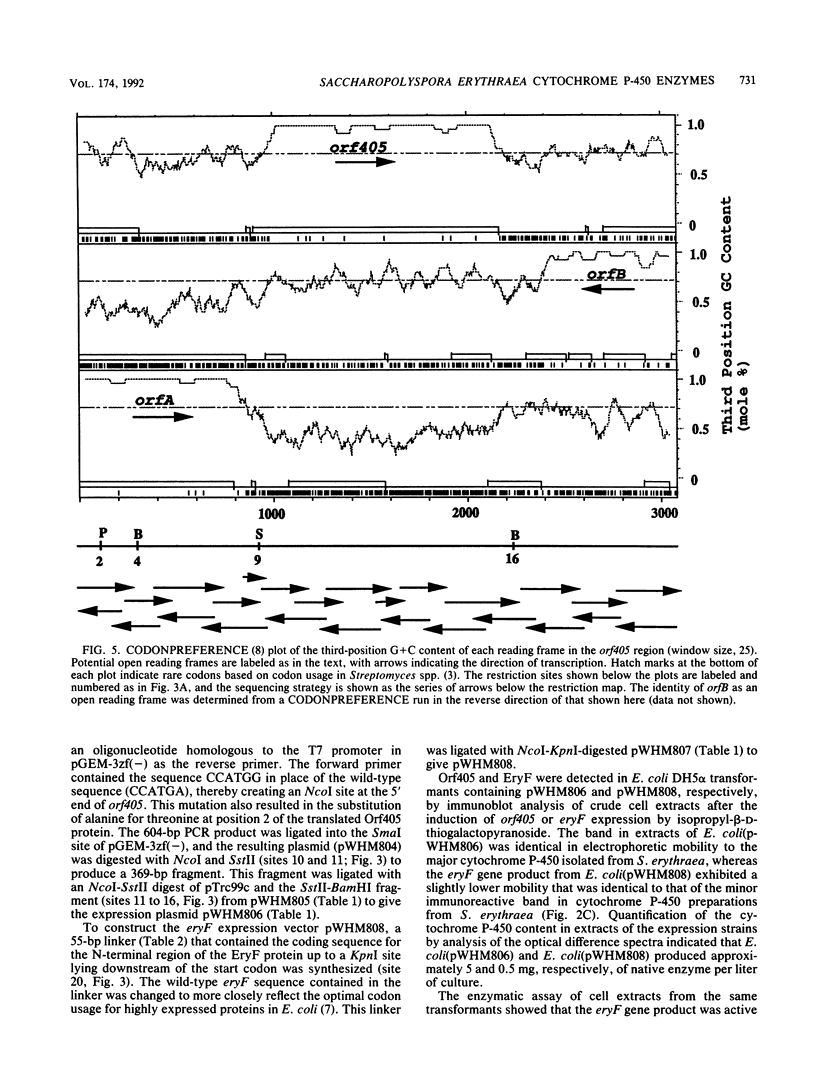
- Donadio S., Hutchinson C. R. Cloning and characterization of the Saccharopolyspora erythraea fdxA gene encoding ferredoxin. Gene. 1991 Apr;100:231–235. doi: 10.1016/0378-1119(91)90372-i. [DOI] [PubMed] [Google Scholar]
- Horii M., Ishizaki T., Paik S. Y., Manome T., Murooka Y. An operon containing the genes for cholesterol oxidase and a cytochrome P-450-like protein from a Streptomyces sp. J Bacteriol. 1990 Jul;172(7):3644–3653. doi: 10.1128/jb.172.7.3644-3653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lancy E. D., Lifsics M. R., Munson P., Maurer R. Nucleotide sequences of dnaE, the gene for the polymerase subunit of DNA polymerase III in Salmonella typhimurium, and a variant that facilitates growth in the absence of another polymerase subunit. J Bacteriol. 1989 Oct;171(10):5581–5586. doi: 10.1128/jb.171.10.5581-5586.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor E. J., Baylis H. A., Chater K. F. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2). Genes Dev. 1987 Dec;1(10):1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- O'Keefe D. P., Gibson K. J., Emptage M. H., Lenstra R., Romesser J. A., Litle P. J., Omer C. A. Ferredoxins from two sulfonylurea herbicide monooxygenase systems in Streptomyces griseolus. Biochemistry. 1991 Jan 15;30(2):447–455. doi: 10.1021/bi00216a021. [DOI] [PubMed] [Google Scholar]
- O'Keefe D. P., Harder P. A. Occurrence and biological function of cytochrome P450 monooxygenases in the actinomycetes. Mol Microbiol. 1991 Sep;5(9):2099–2105. doi: 10.1111/j.1365-2958.1991.tb02139.x. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Omer C. A., Lenstra R., Litle P. J., Dean C., Tepperman J. M., Leto K. J., Romesser J. A., O'Keefe D. P. Genes for two herbicide-inducible cytochromes P-450 from Streptomyces griseolus. J Bacteriol. 1990 Jun;172(6):3335–3345. doi: 10.1128/jb.172.6.3335-3345.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos T. L., Finzel B. C., Howard A. J. High-resolution crystal structure of cytochrome P450cam. J Mol Biol. 1987 Jun 5;195(3):687–700. doi: 10.1016/0022-2836(87)90190-2. [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Kuhstoss S., Solenberg P., Schaus N. A., Rao R. N. A new shuttle cosmid vector, pKC505, for streptomycetes: its use in the cloning of three different spiramycin-resistance genes from a Streptomyces ambofaciens library. Gene. 1987;61(3):231–241. doi: 10.1016/0378-1119(87)90187-9. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S., Rosazza J. P. Novel Biotransformations of 7-Ethoxycoumarin by Streptomyces griseus. Appl Environ Microbiol. 1983 Aug;46(2):468–474. doi: 10.1128/aem.46.2.468-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee A., Hutchinson C. R. Macrolide antibiotic biosynthesis: isolation and properties of two forms of 6-deoxyerythronolide B hydroxylase from Saccharopolyspora erythraea (Streptomyces erythreus). Biochemistry. 1987 Sep 22;26(19):6204–6210. doi: 10.1021/bi00393a037. [DOI] [PubMed] [Google Scholar]
- Shafiee A., Hutchinson C. R. Purification and reconstitution of the electron transport components for 6-deoxyerythronolide B hydroxylase, a cytochrome P-450 enzyme of macrolide antibiotic (erythromycin) biosynthesis. J Bacteriol. 1988 Apr;170(4):1548–1553. doi: 10.1128/jb.170.4.1548-1553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. L., Lalley P. A., Kasper C. B. Chromosomal assignments of genes coding for components of the mixed-function oxidase system in mice. Genetic localization of the cytochrome P-450PCN and P-450PB gene families and the nadph-cytochrome P-450 oxidoreductase and epoxide hydratase genes. J Biol Chem. 1985 Jan 10;260(1):515–521. [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz H. G., McHenry C. S. Sequence analysis of the Escherichia coli dnaE gene. J Bacteriol. 1987 Dec;169(12):5735–5744. doi: 10.1128/jb.169.12.5735-5744.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trower M. K., Sariaslani F. S., O'Keefe D. P. Purification and characterization of a soybean flour-induced cytochrome P-450 from Streptomyces griseus. J Bacteriol. 1989 Apr;171(4):1781–1787. doi: 10.1128/jb.171.4.1781-1787.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara J., Lewandowska-Skarbek M., Wang Y. G., Donadio S., Hutchinson C. R. Cloning of genes governing the deoxysugar portion of the erythromycin biosynthesis pathway in Saccharopolyspora erythraea (Streptomyces erythreus). J Bacteriol. 1989 Nov;171(11):5872–5881. doi: 10.1128/jb.171.11.5872-5881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. M., Leung J. O., Maine G. T., Potenz R. H., Paulus T. J., DeWitt J. P. Organization of a cluster of erythromycin genes in Saccharopolyspora erythraea. J Bacteriol. 1990 May;172(5):2372–2383. doi: 10.1128/jb.172.5.2372-2383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. M., Leung J. O., Swanson S. J., Idler K. B., McAlpine J. B. An erythromycin derivative produced by targeted gene disruption in Saccharopolyspora erythraea. Science. 1991 Apr 5;252(5002):114–117. doi: 10.1126/science.2011746. [DOI] [PubMed] [Google Scholar]
- Weber J. M., Wierman C. K., Hutchinson C. R. Genetic analysis of erythromycin production in Streptomyces erythreus. J Bacteriol. 1985 Oct;164(1):425–433. doi: 10.1128/jb.164.1.425-433.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



