Abstract
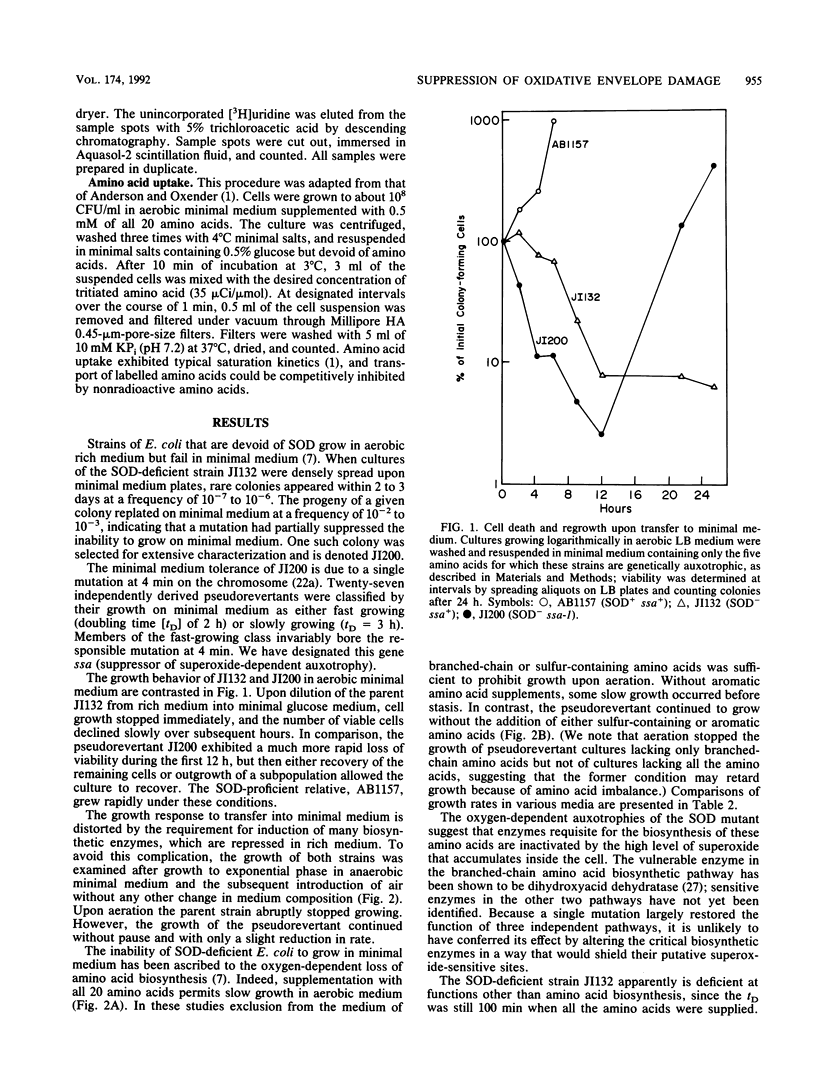
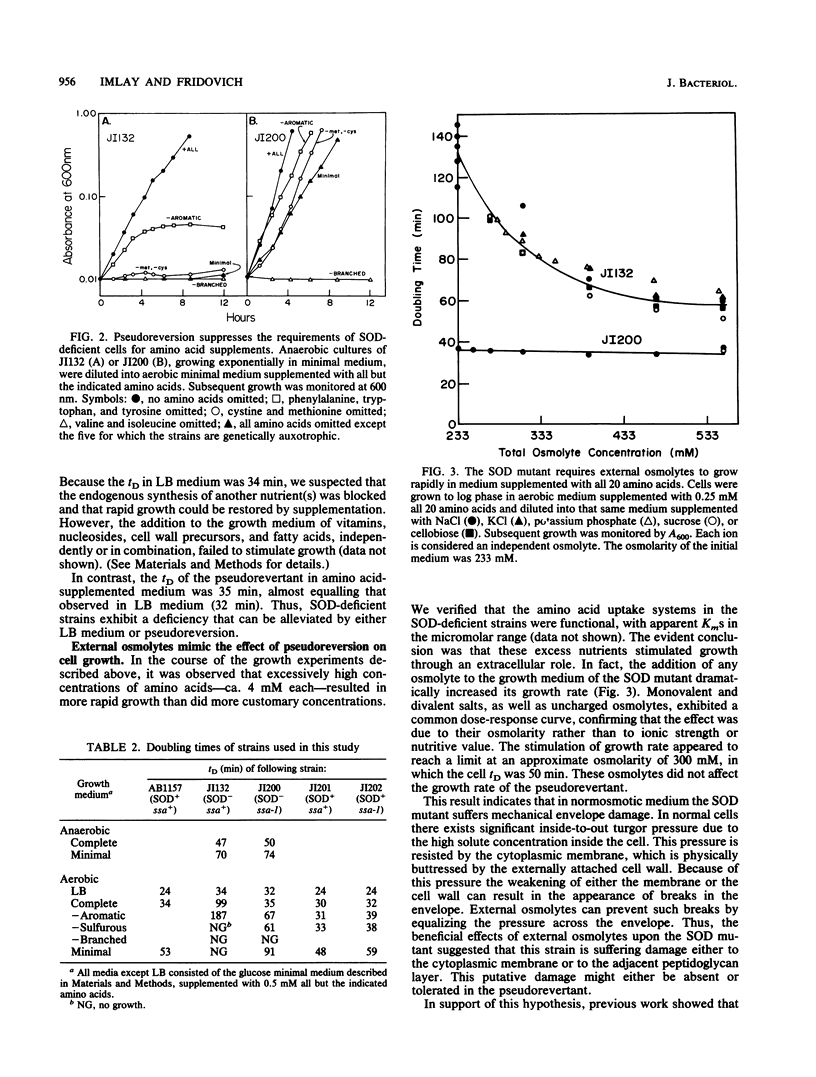
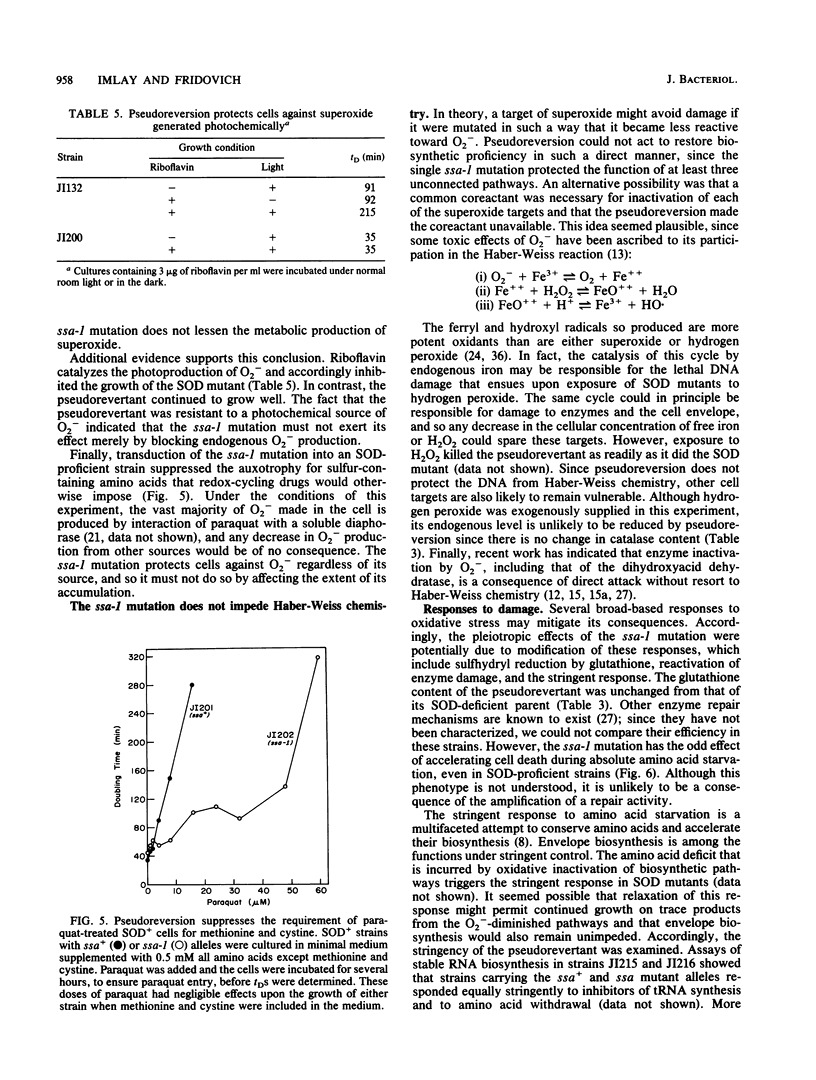
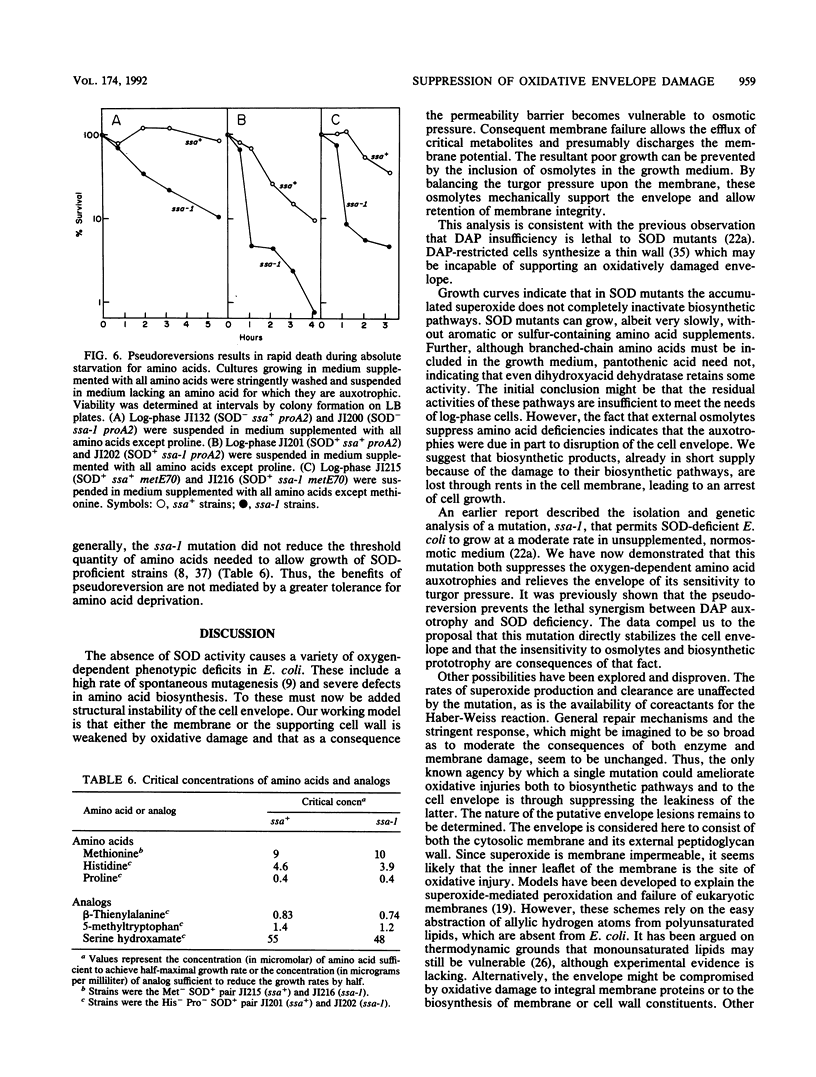
Mutants of Escherichia coli that are devoid of superoxide dismutase (SOD) fail to grow in aerobic minimal medium. This is largely because of the O2- sensitivities of several amino acid biosynthetic pathways, since amino acid supplements can restore growth, albeit at a slow rate. We now report that growth in amino acid-supplemented medium can be further stimulated by the presence of extracellular osmolytes. Osmolytes also partially suppress the amino acid requirements of the SOD mutant. These data suggest that the combination of oxidative injury and turgor pressure permeabilizes the cell envelope and that critical metabolites, including the limiting products of damaged biosynthetic pathways, escape from the cell. External osmolytes may offer protection by countervailing the usual turgor pressure and thus stabilizing the damaged envelope. This model is consistent with the previous observation that deficiency of cell wall components is lethal to SOD mutants. A pseudorevertant that can grow at a moderate rate in normosmotic medium without amino acid supplementation has been obtained (J. A. Imlay and I. Fridovich, Mol. Gen. Genet. 228:410-416, 1991). Analysis suggests that the suppressor mutation allows the envelope either to resist or to tolerate oxidative lesions. Study of the pseudorevertant may illuminate the molecular basis of this oxidative envelope injury.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Oxender D. L. Genetic separation of high- and low-affinity transport systems for branched-chain amino acids in Escherichia coli K-12. J Bacteriol. 1978 Oct;136(1):168–174. doi: 10.1128/jb.136.1.168-174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981 Jan;145(1):442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biliński T., Krawiec Z., Liczmański A., Litwińska J. Is hydroxyl radical generated by the Fenton reaction in vivo? Biochem Biophys Res Commun. 1985 Jul 31;130(2):533–539. doi: 10.1016/0006-291x(85)90449-8. [DOI] [PubMed] [Google Scholar]
- Bowler C., Alliotte T., Van den Bulcke M., Bauw G., Vandekerckhove J., Van Montagu M., Inzé D. A plant manganese superoxide dismutase is efficiently imported and correctly processed by yeast mitochondria. Proc Natl Acad Sci U S A. 1989 May;86(9):3237–3241. doi: 10.1073/pnas.86.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C., Van Kaer L., Van Camp W., Van Montagu M., Inzé D., Dhaese P. Characterization of the Bacillus stearothermophilus manganese superoxide dismutase gene and its ability to complement copper/zinc superoxide dismutase deficiency in Saccharomyces cerevisiae. J Bacteriol. 1990 Mar;172(3):1539–1546. doi: 10.1128/jb.172.3.1539-1546.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Touati D., Kogoma T. Effects of oxygen stress on membrane functions in Escherichia coli: role of HPI catalase. J Bacteriol. 1988 Apr;170(4):1837–1842. doi: 10.1128/jb.170.4.1837-1842.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989 May 15;264(14):7761–7764. [PubMed] [Google Scholar]
- Ganong B. R., Raetz C. R. Massive accumulation of phosphatidic acid in conditionally lethal CDP-diglyceride synthetase mutants and cytidine auxotrophs of Escherichia coli. J Biol Chem. 1982 Jan 10;257(1):389–394. [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J Biol Chem. 1991 Jan 25;266(3):1478–1483. [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991 Oct 15;266(29):19328–19333. [PubMed] [Google Scholar]
- Green M. J., Hill H. A. Chemistry of dioxygen. Methods Enzymol. 1984;105:3–22. doi: 10.1016/s0076-6879(84)05004-7. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol. 1986 Nov;168(2):1026–1029. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber M. Y., Glick B. R., Thompson J. E. Cloned manganese superoxide dismutase reduces oxidative stress in Escherichia coli and Anacystis nidulans. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2608–2612. doi: 10.1073/pnas.87.7.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990 Apr;15(4):129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991 Apr 15;266(11):6957–6965. [PubMed] [Google Scholar]
- Imlay J. A., Fridovich I. Isolation and genetic analysis of a mutation that suppresses the auxotrophies of superoxide dismutase-deficient Escherichia coli K12. Mol Gen Genet. 1991 Sep;228(3):410–416. doi: 10.1007/BF00260634. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J Bacteriol. 1987 Jul;169(7):2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen R., Chevion M. Cytoplasmic membrane is the target organelle for transition metal mediated damage induced by paraquat in Escherichia coli. Biochemistry. 1988 Apr 5;27(7):2597–2603. doi: 10.1021/bi00407a049. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H. Oxyradical reactions: from bond-dissociation energies to reduction potentials. FEBS Lett. 1990 May 21;264(2):165–167. doi: 10.1016/0014-5793(90)80239-f. [DOI] [PubMed] [Google Scholar]
- Kuo C. F., Mashino T., Fridovich I. alpha, beta-Dihydroxyisovalerate dehydratase. A superoxide-sensitive enzyme. J Biol Chem. 1987 Apr 5;262(10):4724–4727. [PubMed] [Google Scholar]
- Levén S., Heimberger A., Eisenstark A. Catalase HPI influences membrane permeability in Escherichia coli following near-UV stress. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1224–1228. doi: 10.1016/0006-291x(90)90816-6. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natvig D. O., Imlay K., Touati D., Hallewell R. A. Human copper-zinc superoxide dismutase complements superoxide dismutase-deficient Escherichia coli mutants. J Biol Chem. 1987 Oct 25;262(30):14697–14701. [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats R., de Pedro M. A. Normal growth and division of Escherichia coli with a reduced amount of murein. J Bacteriol. 1989 Jul;171(7):3740–3745. doi: 10.1128/jb.171.7.3740-3745.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnock G., Wild D. G. Synthesis of ribonucleic acid and protein during inhibition of Escherichia coli by analogues of amino acids. Biochim Biophys Acta. 1966 Aug 17;123(2):402–415. doi: 10.1016/0005-2787(66)90292-9. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Larson R. A., Kagan J. Role of cloned carotenoid genes expressed in Escherichia coli in protecting against inactivation by near-UV light and specific phototoxic molecules. J Bacteriol. 1988 Oct;170(10):4675–4680. doi: 10.1128/jb.170.10.4675-4680.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp W., Bowler C., Villarroel R., Tsang E. W., Van Montagu M., Inzé D. Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9903–9907. doi: 10.1073/pnas.87.24.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein B. I., Bhardwaj N., Li H. C. A rapid and accurate procedure for assaying DNA polymerase, RNA polymerase, or ribosome dependent protein synthesis. Anal Biochem. 1975 Sep;68(1):62–69. doi: 10.1016/0003-2697(75)90679-x. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., Pesold-Hurt B., Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]



