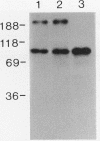
Abstract
We have investigated a tyrosine kinase activity from Pseudomonas solanacearum, an economically important plant pathogen. In vitro incubation of membrane fractions with [gamma-32P]ATP and subsequent sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed an 85-kDa phosphoprotein. Phosphorylation of this protein on tyrosine residues was demonstrated by phosphoamino acid analysis of base hydrolysis products and by immunoanalysis of Western blots (immunoblots) with antiphosphotyrosine monoclonal antibody. In vitro incubation of membranes with ATP was not required for recognition by the antibody, indicating that the 85-kDa protein is phosphorylated in vivo. These results demonstrate that membranes from P. solanacearum exhibit a tyrosine kinase activity toward an endogenous membrane protein. This bacterium provides an opportunity to study the structure and function of a prokaryotic tyrosine kinase.
Full text
PDF
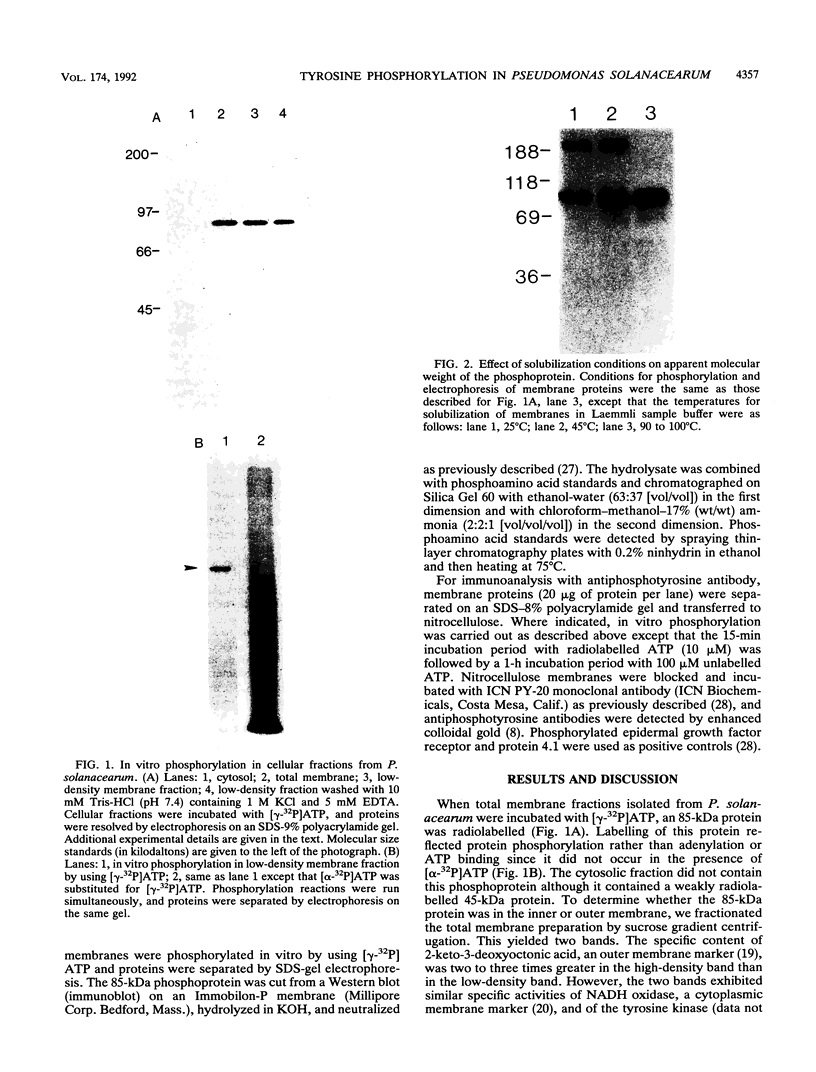


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook D., Sequeira L. Genetic and biochemical characterization of a Pseudomonas solanacearum gene cluster required for extracellular polysaccharide production and for virulence. J Bacteriol. 1991 Mar;173(5):1654–1662. doi: 10.1128/jb.173.5.1654-1662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortay J. C., Rieul C., Duclos B., Cozzone A. J. Characterization of the phosphoproteins of Escherichia coli cells by electrophoretic analysis. Eur J Biochem. 1986 Sep 1;159(2):227–237. doi: 10.1111/j.1432-1033.1986.tb09858.x. [DOI] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Dadssi M., Cozzone A. J. Evidence of protein-tyrosine kinase activity in the bacterium Acinetobacter calcoaceticus. J Biol Chem. 1990 Dec 5;265(34):20996–20999. [PubMed] [Google Scholar]
- Dadssi M., Cozzone A. J. Occurrence of protein phosphorylation in various bacterial species. Int J Biochem. 1990;22(5):493–499. doi: 10.1016/0020-711x(90)90263-3. [DOI] [PubMed] [Google Scholar]
- Danscher G., Nörgaard J. O. Light microscopic visualization of colloidal gold on resin-embedded tissue. J Histochem Cytochem. 1983 Dec;31(12):1394–1398. doi: 10.1177/31.12.6631001. [DOI] [PubMed] [Google Scholar]
- Foster R., Thorner J., Martin G. S. Nucleotidylation, not phosphorylation, is the major source of the phosphotyrosine detected in enteric bacteria. J Bacteriol. 1989 Jan;171(1):272–279. doi: 10.1128/jb.171.1.272-279.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick C. A., Sequeira L. Lipopolysaccharide-Defective Mutants of the Wilt Pathogen Pseudomonas solanacearum. Appl Environ Microbiol. 1984 Jul;48(1):94–101. doi: 10.1128/aem.48.1.94-101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Matsumura P., Simon M. I. Protein phosphorylation is involved in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7609–7613. doi: 10.1073/pnas.84.21.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Wintenberg K., Anderson T., Montie T. C. Phosphorylated tyrosine in the flagellum filament protein of Pseudomonas aeruginosa. J Bacteriol. 1990 Sep;172(9):5135–5139. doi: 10.1128/jb.172.9.5135-5139.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Londesborough J. Phosphorylation of proteins in Clostridium thermohydrosulfuricum. J Bacteriol. 1986 Feb;165(2):595–601. doi: 10.1128/jb.165.2.595-601.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaï M., Cozzone A. J. Endogenous protein phosphorylation in Escherichia coli extracts. Biochem Biophys Res Commun. 1982 Aug;107(3):981–988. doi: 10.1016/0006-291x(82)90619-2. [DOI] [PubMed] [Google Scholar]
- Martensen T. M. Chemical properties, isolation, and analysis of O-phosphates in proteins. Methods Enzymol. 1984;107:3–23. doi: 10.1016/0076-6879(84)07003-8. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pairoba C. F., Vallejos R. H. Protein phosphorylation in purple photosynthetic bacteria. Biochimie. 1989 Sep-Oct;71(9-10):1039–1041. doi: 10.1016/0300-9084(89)90108-9. [DOI] [PubMed] [Google Scholar]
- Roberts D. P., Denny T. P., Schell M. A. Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. J Bacteriol. 1988 Apr;170(4):1445–1451. doi: 10.1128/jb.170.4.1445-1451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Wu L. F., Reizer J. Regulation of bacterial physiological processes by three types of protein phosphorylating systems. Trends Biochem Sci. 1990 Oct;15(10):391–395. doi: 10.1016/0968-0004(90)90238-7. [DOI] [PubMed] [Google Scholar]
- Schieven G., Thorner J., Martin G. S. Protein-tyrosine kinase activity in Saccharomyces cerevisiae. Science. 1986 Jan 24;231(4736):390–393. doi: 10.1126/science.2417318. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Halpern R. M., Bruegger B. B., Dunlap A. K., Fricke O. Chromosomal protein phosphorylation on basic amino acids. Methods Cell Biol. 1978;19:153–159. doi: 10.1016/s0091-679x(08)60020-5. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam G., Bertics P. J., Anderson R. A. Phosphorylation of protein 4.1 on tyrosine-418 modulates its function in vitro. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5222–5226. doi: 10.1073/pnas.88.12.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejos R. H., Holuigue L., Lucero H. A., Torruella M. Evidence of tyrosine kinase activity in the photosynthetic bacterium Rhodospirillum rubrum. Biochem Biophys Res Commun. 1985 Jan 31;126(2):685–691. doi: 10.1016/0006-291x(85)90239-6. [DOI] [PubMed] [Google Scholar]