Abstract
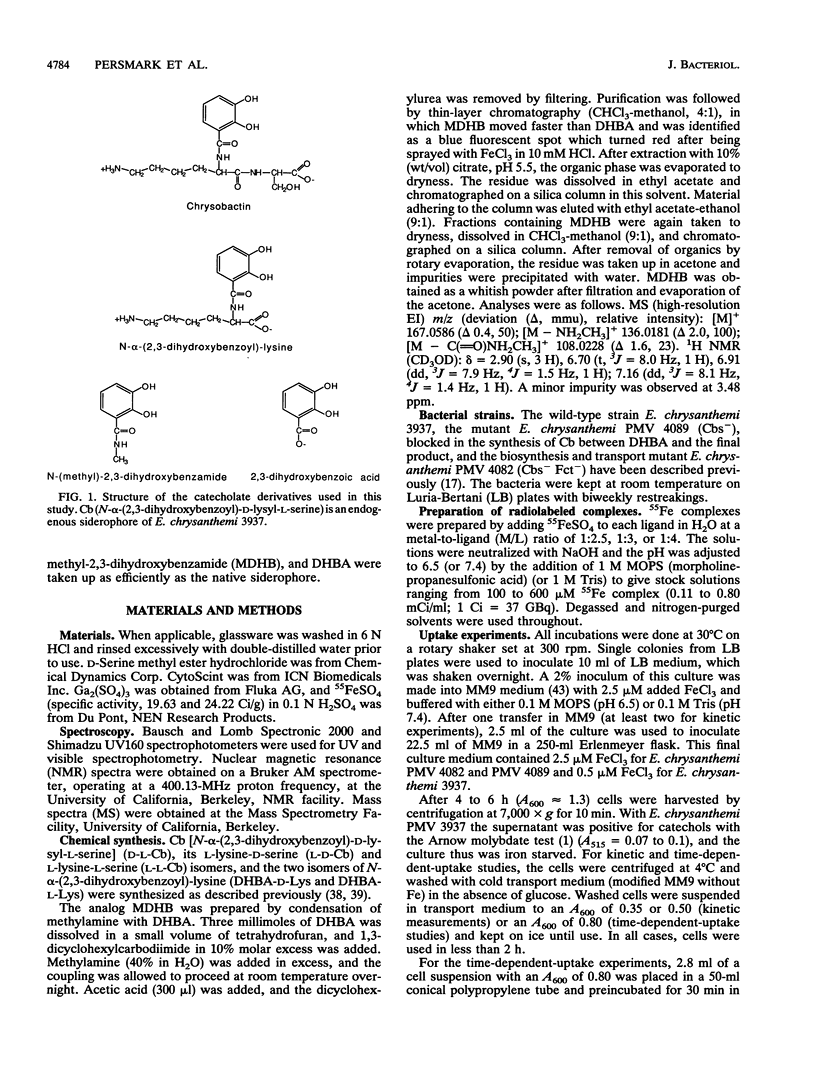
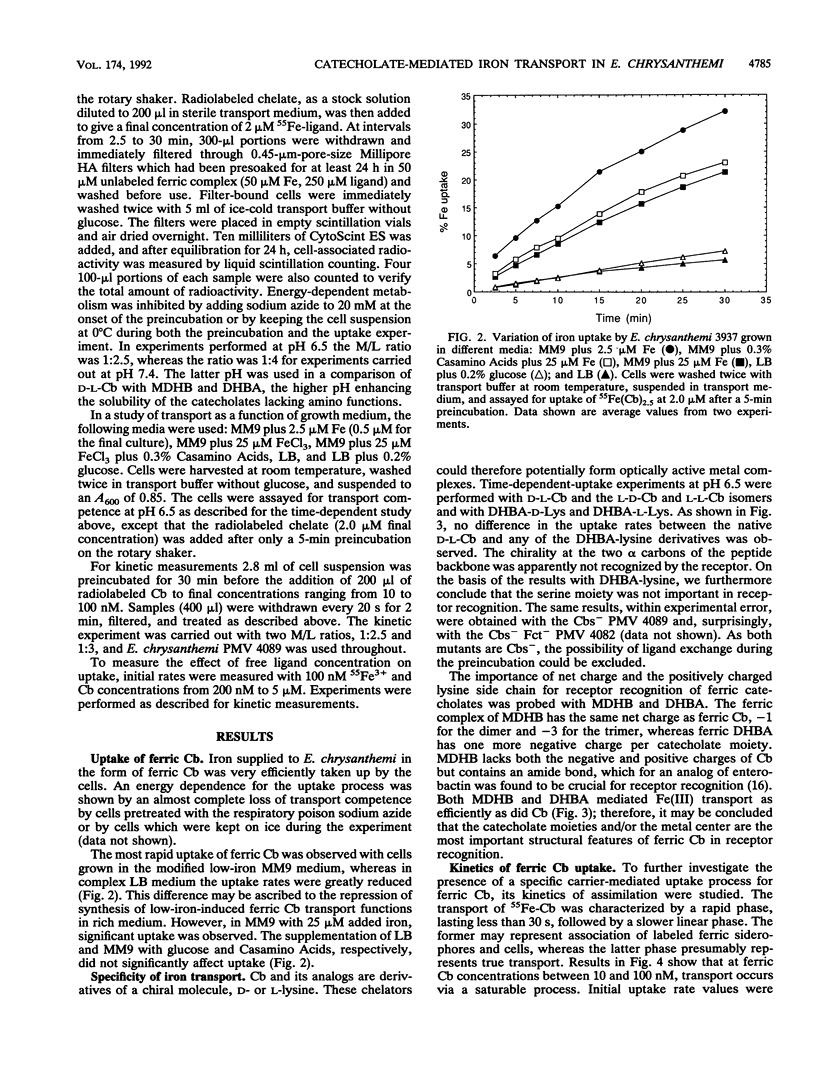
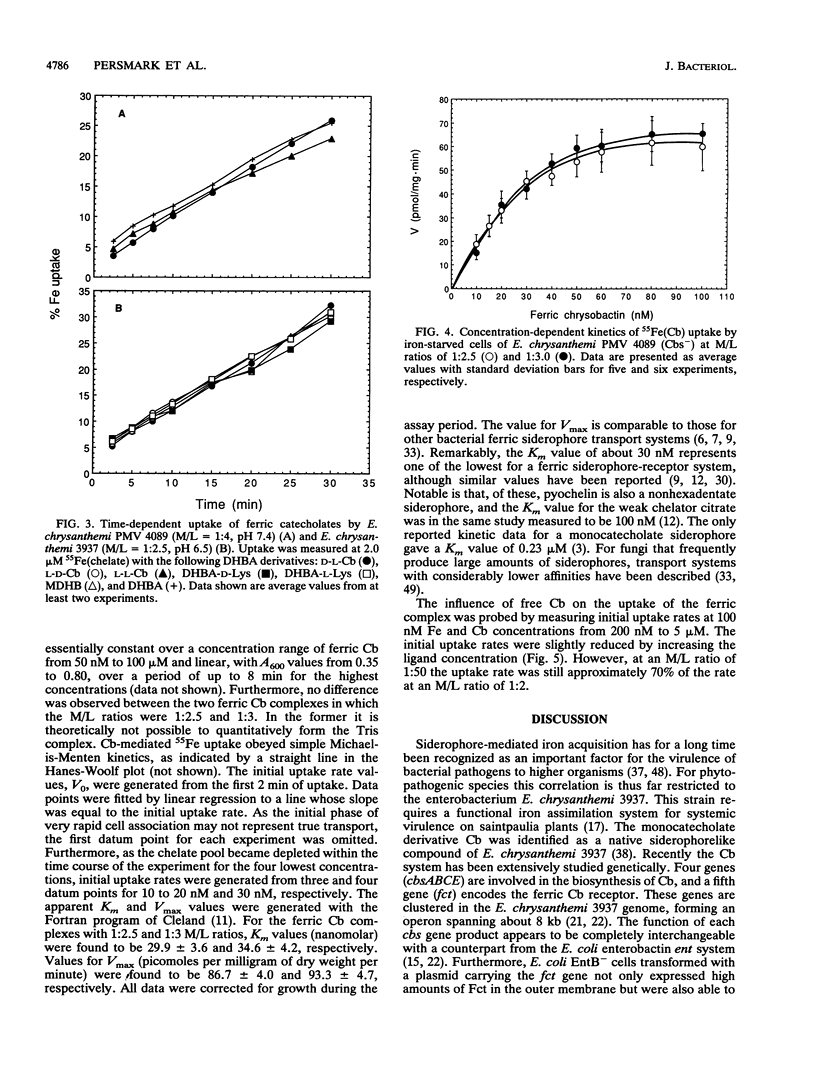
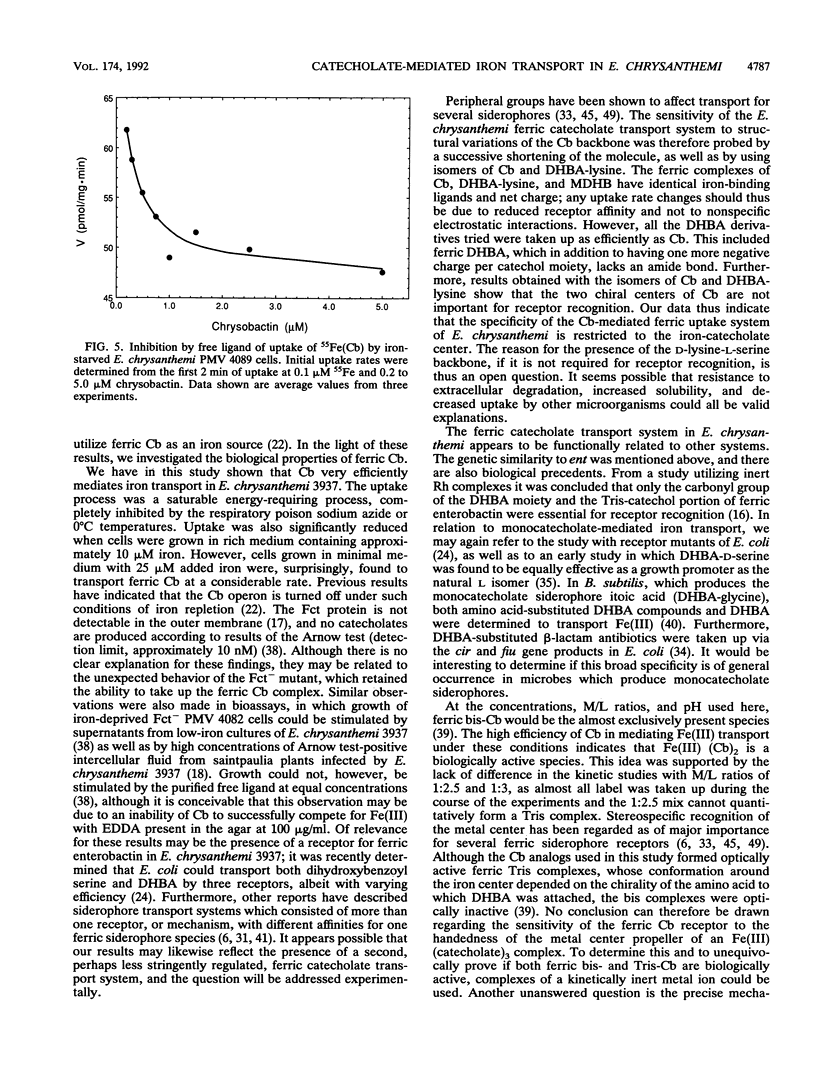
Erwinia chrysanthemi 3937 possesses a saturable, high-affinity transport system for the ferric complex of its native siderophore chrysobactin, [N-alpha-(2,3-dihydroxybenzoyl)-D-lysyl-L-serine]. Uptake of 55Fe-labeled chrysobactin was completely inhibited by respiratory poison or low temperature and was significantly reduced in rich medium. The kinetics of chrysobactin-mediated iron transport were determined to have apparent Km and Vmax values of about 30 nM and of 90 pmol/mg.min, respectively. Isomers of chrysobactin and analogs with progressively shorter side chains mediated ferric iron transport as efficiently as the native siderophore, which indicates that the chrysobactin receptor primarily recognizes the catechol-iron center. Free ligand in excess only moderately reduced the accumulation of 55Fe. Chrysobactin may therefore be regarded as a true siderophore for E. chrysanthemi.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin C. L., Neilands J. B. Rhodotorulic acid, a diketopiperazine dihydroxamic acid with growth-factor activity. I. Isolation and characterization. Biochemistry. 1968 Oct;7(10):3734–3739. doi: 10.1021/bi00850a054. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghouthi S., Young R., Olson M. O., Arceneaux J. E., Clem L. W., Byers B. R. Amonabactin, a novel tryptophan- or phenylalanine-containing phenolate siderophore in Aeromonas hydrophila. J Bacteriol. 1989 Apr;171(4):1811–1816. doi: 10.1128/jb.171.4.1811-1816.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron R. J., Weimar W. R. Kinetics of iron acquisition from ferric siderophores by Paracoccus denitrificans. J Bacteriol. 1990 May;172(5):2650–2657. doi: 10.1128/jb.172.5.2650-2657.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner I., Konetschny-Rapp S., Jung G., Winkelmann G. Characterization of ferrioxamine E as the principal siderophore of Erwinia herbicola (Enterobacter agglomerans). Biol Met. 1988;1(1):51–56. doi: 10.1007/BF01128017. [DOI] [PubMed] [Google Scholar]
- Berner I., Winkelmann G. Ferrioxamine transport mutants and the identification of the ferrioxamine receptor protein (FoxA) in Erwinia herbicola (Enterobacter agglomerans). Biol Met. 1990;2(4):197–202. doi: 10.1007/BF01141359. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Rinehart K. L., Jr, Moore M. L., Cook J. C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann H. Stoffwechselprodukte von Mikroorganismen. 81. Vorkommen und Strukturen von Coprogen B und Dimerumsäure. Arch Mikrobiol. 1970;73(1):65–76. [PubMed] [Google Scholar]
- Enard C., Diolez A., Expert D. Systemic virulence of Erwinia chrysanthemi 3937 requires a functional iron assimilation system. J Bacteriol. 1988 Jun;170(6):2419–2426. doi: 10.1128/jb.170.6.2419-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert D., Toussaint A. Bacteriocin-resistant mutants of Erwinia chrysanthemi: possible involvement of iron acquisition in phytopathogenicity. J Bacteriol. 1985 Jul;163(1):221–227. doi: 10.1128/jb.163.1.221-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S., Cooper R. A., Evans R. W., Hider R. C., Williams P. H. Domain preference in iron removal from human transferrin by the bacterial siderophores aerobactin and enterochelin. Eur J Biochem. 1988 Dec 15;178(2):477–481. doi: 10.1111/j.1432-1033.1988.tb14473.x. [DOI] [PubMed] [Google Scholar]
- Franza T., Enard C., van Gijsegem F., Expert D. Genetic analysis of the Erwinia chrysanthemi 3937 chrysobactin iron-transport system: characterization of a gene cluster involved in uptake and biosynthetic pathways. Mol Microbiol. 1991 Jun;5(6):1319–1329. doi: 10.1111/j.1365-2958.1991.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Franza T., Expert D. The virulence-associated chrysobactin iron uptake system of Erwinia chrysanthemi 3937 involves an operon encoding transport and biosynthetic functions. J Bacteriol. 1991 Nov;173(21):6874–6881. doi: 10.1128/jb.173.21.6874-6881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Dihydroxybenzoylserine--a siderophore for E. coli. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):5–8. doi: 10.1016/0378-1097(90)90158-m. [DOI] [PubMed] [Google Scholar]
- Jalal M. A., Love S. K., van der Helm D. Siderophore mediated iron(III) uptake in Gliocladium virens. 2. Role of ferric mono- and dihydroxamates as iron transport agents. J Inorg Biochem. 1987 Apr;29(4):259–267. doi: 10.1016/0162-0134(87)80033-8. [DOI] [PubMed] [Google Scholar]
- Knosp O., von Tigerstrom M., Page W. J. Siderophore-mediated uptake of iron in Azotobacter vinelandii. J Bacteriol. 1984 Jul;159(1):341–347. doi: 10.1128/jb.159.1.341-347.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobaru S., Tsunakawa M., Hanada M., Konishi M., Tomita K., Kawaguchi H. Bu-2743E, a leucine aminopeptidase inhibitor, produced by Bacillus circulans. J Antibiot (Tokyo) 1983 Oct;36(10):1396–1398. doi: 10.7164/antibiotics.36.1396. [DOI] [PubMed] [Google Scholar]
- Kunze B., Bedorf N., Kohl W., Höfle G., Reichenbach H. Myxochelin A, a new iron-chelating compound from Angiococcus disciformis (Myxobacterales). Production, isolation, physico-chemical and biological properties. J Antibiot (Tokyo) 1989 Jan;42(1):14–17. doi: 10.7164/antibiotics.42.14. [DOI] [PubMed] [Google Scholar]
- Lammers P. J., Sanders-Loehr J. Active transport of ferric schizokinen in Anabaena sp. J Bacteriol. 1982 Jul;151(1):288–294. doi: 10.1128/jb.151.1.288-294.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Cir and Fiu proteins in the outer membrane of Escherichia coli catalyze transport of monomeric catechols: study with beta-lactam antibiotics containing catechol and analogous groups. J Bacteriol. 1990 Mar;172(3):1361–1367. doi: 10.1128/jb.172.3.1361-1367.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien I. G., Cox G. B., Gibson F. 2,3-dihydroxy-N-benzoylserine: chemical synthesis and comparison with the natural product. Biochim Biophys Acta. 1969 Apr 1;177(2):321–328. doi: 10.1016/0304-4165(69)90142-1. [DOI] [PubMed] [Google Scholar]
- Payne S. M. Iron and virulence in the family Enterobacteriaceae. Crit Rev Microbiol. 1988;16(2):81–111. doi: 10.3109/10408418809104468. [DOI] [PubMed] [Google Scholar]
- Persmark M., Expert D., Neilands J. B. Isolation, characterization, and synthesis of chrysobactin, a compound with siderophore activity from Erwinia chrysanthemi. J Biol Chem. 1989 Feb 25;264(6):3187–3193. [PubMed] [Google Scholar]
- Persmark M., Neilands J. B. Iron(III) complexes of chrysobactin, the siderophore of Erwinia chrysanthemi. Biometals. 1992 Spring;5(1):29–36. doi: 10.1007/BF01079695. [DOI] [PubMed] [Google Scholar]
- Peters W. J., Warren R. A. The mechanism of iron uptake in Bacillus subtilis. Can J Microbiol. 1970 Dec;16(12):1285–1291. doi: 10.1139/m70-214. [DOI] [PubMed] [Google Scholar]
- Poole K., Young L., Neshat S. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol. 1990 Dec;172(12):6991–6996. doi: 10.1128/jb.172.12.6991-6996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Skorupska A., Choma A., Deryło M., Lorkiewicz Z. Siderophore containing 2,3-dihydroxybenzoic acid and threonine formed by Rhizobium trifolli. Acta Biochim Pol. 1988;35(2):119–130. [PubMed] [Google Scholar]
- Van Hove B., Staudenmaier H., Braun V. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J Bacteriol. 1990 Dec;172(12):6749–6758. doi: 10.1128/jb.172.12.6749-6758.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Newton A. Iron transport in Escherichia coli: roles of energy-dependent uptake and 2,3-dihydroxybenzoylserine. J Bacteriol. 1969 Jun;98(3):1142–1150. doi: 10.1128/jb.98.3.1142-1150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]


