Abstract
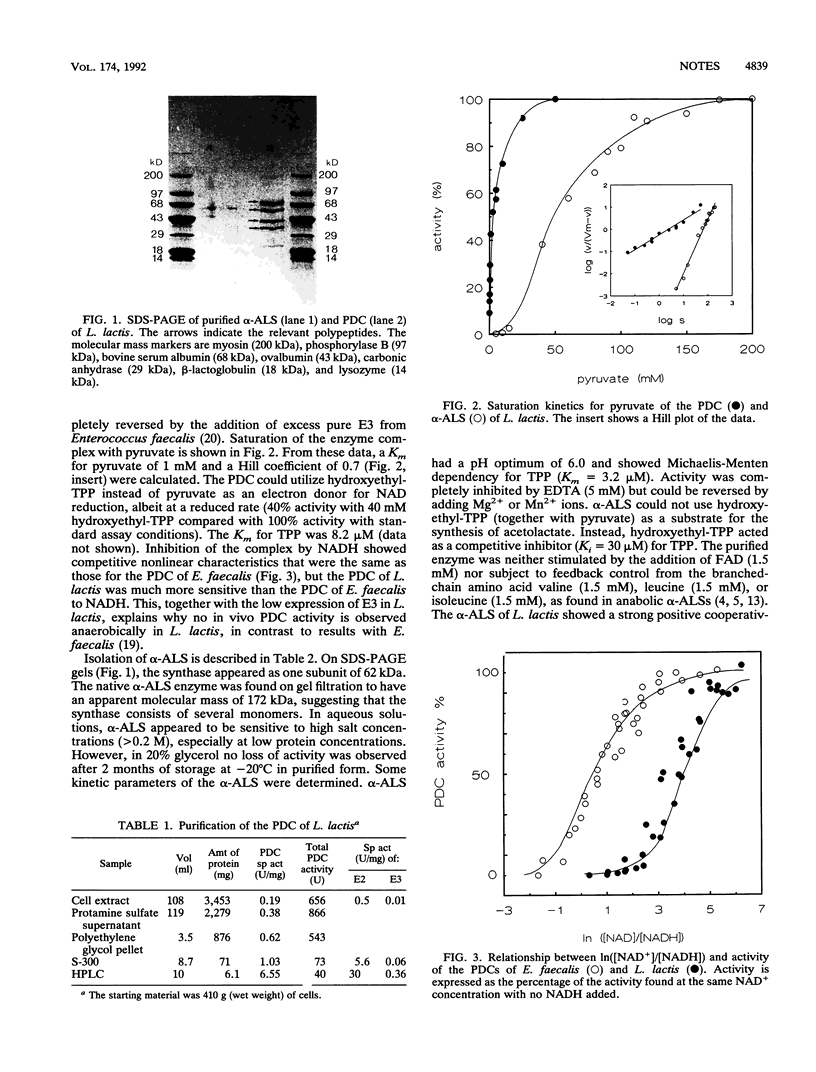
The pyruvate dehydrogenase complex of Lactococcus lactis subsp. lactis bv. diacetylactis has a specific activity of 6.6 U/mg and a Km of 1 mM for pyruvate. The specific activities of E2 and E3 in the complex are 30 and 0.36 U/mg, respectively. The complex is very sensitive to NADH inhibition and consists of four subunits: E1 alpha (44 kDa), E1 beta (35 kDa), E2 (73 kDa), and E3 (60 kDa). The L. lactis alpha-acetolactate synthase has a specific activity of 103 U/mg and a Km of 50 mM for pyruvate. Thiamine pyrophosphate (Km = 3.2 microM) and divalent cations are essential for activity. The native enzyme measures 172 kDa and consists of 62-kDa monomers. The role of both enzymes in product formation is discussed in view of NADH inhibition and competition for pyruvate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Durner J., Böger P. Oligomeric forms of plant acetolactate synthase depend on flavin adenine dinucleotide. Plant Physiol. 1990 Jul;93(3):1027–1031. doi: 10.1104/pp.93.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):184–189. doi: 10.1128/jb.157.1.184-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruys K. J., Halkides C. J., Frey P. A. Synthesis and properties of 2-acetylthiamin pyrophosphate: an enzymatic reaction intermediate. Biochemistry. 1987 Dec 1;26(24):7575–7585. doi: 10.1021/bi00398a007. [DOI] [PubMed] [Google Scholar]
- Hawkins C. F., Borges A., Perham R. N. A common structural motif in thiamin pyrophosphate-binding enzymes. FEBS Lett. 1989 Sep 11;255(1):77–82. doi: 10.1016/0014-5793(89)81064-6. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Perham R. N. Purificaton of the pyruvate dehydrogenase multienzyme complex of Bacillus stearothermophilus and resolution of its four component polypeptides. Biochem J. 1980 Jul 1;189(1):161–172. doi: 10.1042/bj1890161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzclaw W. D., Chapman L. F. Degradative acetolactate synthase of Bacillus subtilis: purification and properties. J Bacteriol. 1975 Mar;121(3):917–922. doi: 10.1128/jb.121.3.917-922.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E. Mechanisms of the formation of acetoin by yeast and mammalian tissue. J Biol Chem. 1952 Apr;195(2):727–734. [PubMed] [Google Scholar]
- Kaneko T., Takahashi M., Suzuki H. Acetoin Fermentation by Citrate-Positive Lactococcus lactis subsp. lactis 3022 Grown Aerobically in the Presence of Hemin or Cu. Appl Environ Microbiol. 1990 Sep;56(9):2644–2649. doi: 10.1128/aem.56.9.2644-2649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthe-Sorenssen D., Stormer F. C. The pH 6 acetolactate-forming enzyme from Serratia marcescens. Purification and properties. Eur J Biochem. 1970 May 1;14(1):127–132. doi: 10.1111/j.1432-1033.1970.tb00269.x. [DOI] [PubMed] [Google Scholar]
- SCHREIBER G., KOHLHAW G., GOEDDE H. W., HOLZER H. DIE BIOSYNTHESE VON ACETOIN IN SCHWEINEHERZMUSKEL. Biochem Z. 1963 Oct 14;339:83–93. [PubMed] [Google Scholar]
- Schwartz E. R., Reed L. J. Alpha-keto acid dehydrogenase complexes. XII. Effects of acetylation on the activity and structure of the dihydrolipoyl transacetylase of Escherichia coli. J Biol Chem. 1969 Nov 25;244(22):6074–6079. [PubMed] [Google Scholar]
- Schwartz E. R., Reed L. J. Regulation of the activity of the pyruvate dehydrogenase complex of Escherichia coli. Biochemistry. 1970 Mar 17;9(6):1434–1439. doi: 10.1021/bi00808a019. [DOI] [PubMed] [Google Scholar]
- Snoep J. L., Teixeira de Mattos M. J., Postma P. W., Neijssel O. M. Involvement of pyruvate dehydrogenase in product formation in pyruvate-limited anaerobic chemostat cultures of Enterococcus faecalis NCTC 775. Arch Microbiol. 1990;154(1):50–55. doi: 10.1007/BF00249177. [DOI] [PubMed] [Google Scholar]
- Snoep J. L., Westphal A. H., Benen J. A., Teixeira de Mattos M. J., Neijssel O. M., de Kok A. Isolation and characterisation of the pyruvate dehydrogenase complex of anaerobically grown Enterococcus faecalis NCTC 775. Eur J Biochem. 1992 Jan 15;203(1-2):245–250. doi: 10.1111/j.1432-1033.1992.tb19853.x. [DOI] [PubMed] [Google Scholar]
- Starrenburg M. J., Hugenholtz J. Citrate Fermentation by Lactococcus and Leuconostoc spp. Appl Environ Microbiol. 1991 Dec;57(12):3535–3540. doi: 10.1128/aem.57.12.3535-3540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhue W. M., Tjan F. S. Study of the Citrate Metabolism of Lactococcus lactis subsp. lactis Biovar Diacetylactis by Means of C Nuclear Magnetic Resonance. Appl Environ Microbiol. 1991 Nov;57(11):3371–3377. doi: 10.1128/aem.57.11.3371-3377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal A. H., de Kok A. Lipoamide dehydrogenase from Azotobacter vinelandii. Molecular cloning, organization and sequence analysis of the gene. Eur J Biochem. 1988 Mar 1;172(2):299–305. doi: 10.1111/j.1432-1033.1988.tb13887.x. [DOI] [PubMed] [Google Scholar]