Abstract
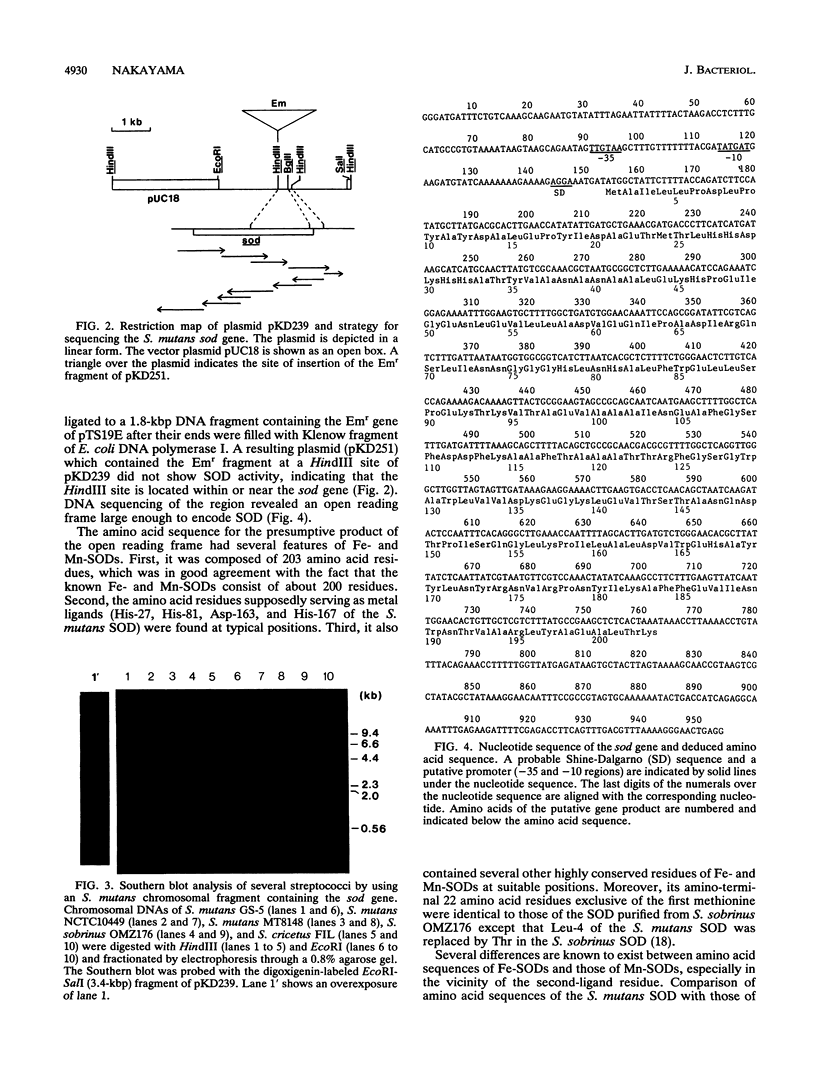
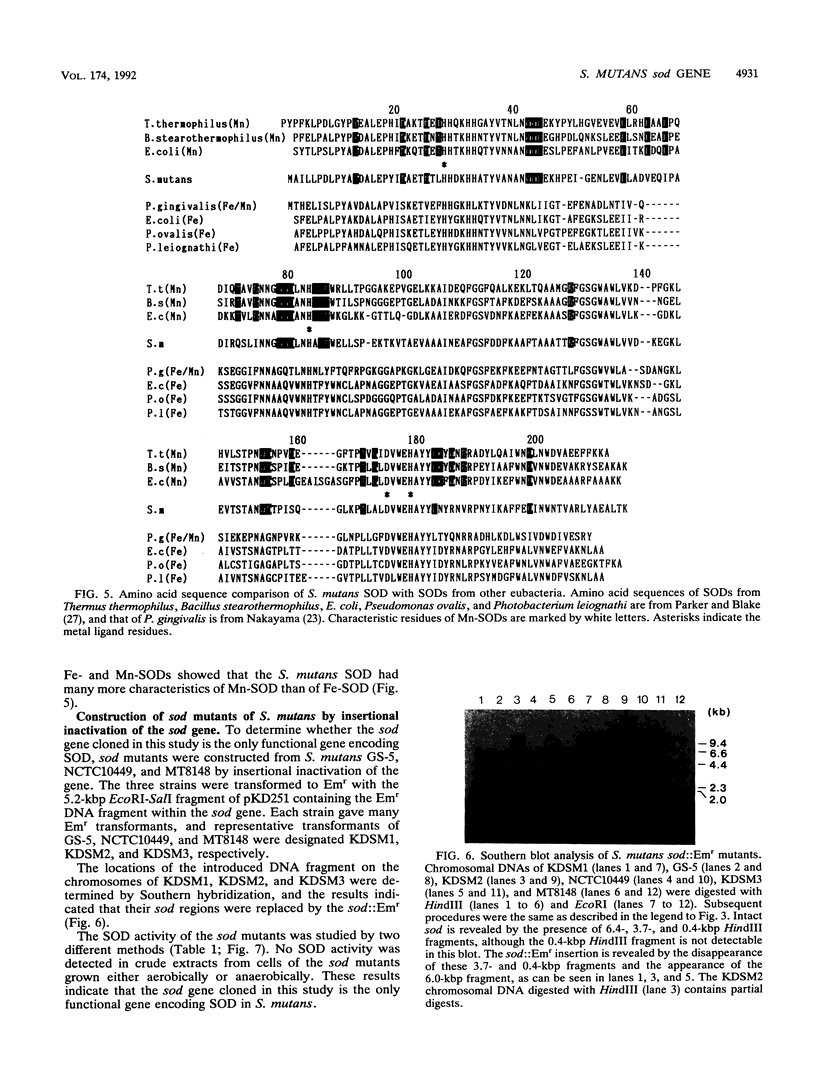
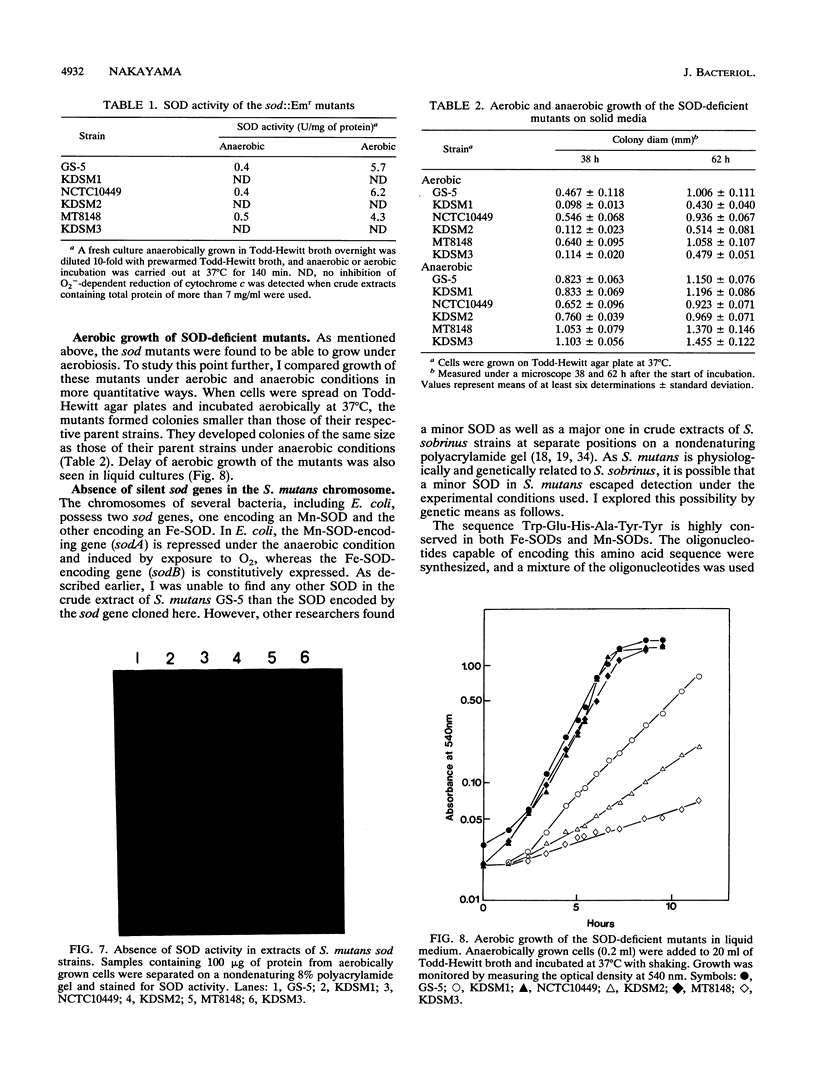
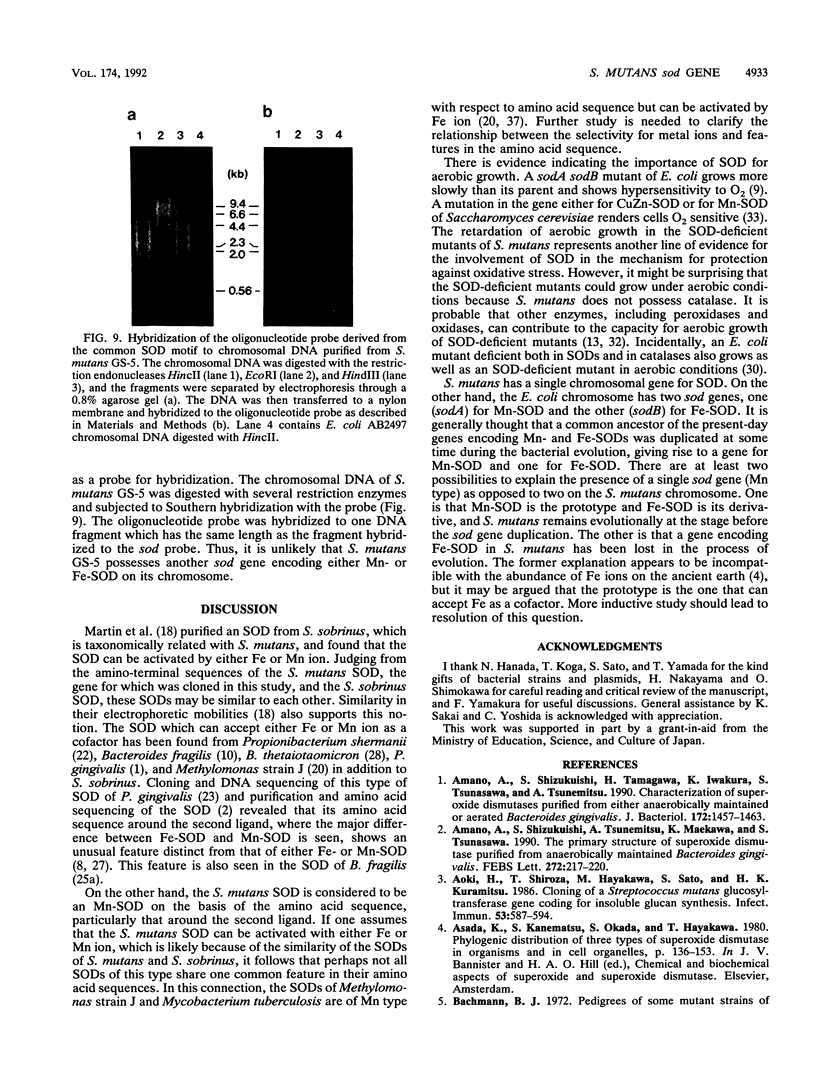
A gene (sod) encoding superoxide dismutase (SOD) was cloned from Streptococcus mutans in Escherichia coli, and its nucleotide sequence was determined. The presumptive amino acid sequence of its product revealed that the SOD is basically of Mn type. Insertional inactivation of the sod gene resulted in the loss of SOD activity in crude extracts, indicating that the gene represents the only functional gene for SOD in S. mutans. Moreover, Southern blot analysis indicated that the S. mutans chromosome had no additional gene which was hybridizable with an oligonucleotide probe specific for an SOD motif. The SOD-deficient mutants were able to grow aerobically, albeit more slowly than the parent strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano A., Shizukuishi S., Tamagawa H., Iwakura K., Tsunasawa S., Tsunemitsu A. Characterization of superoxide dismutases purified from either anaerobically maintained or aerated Bacteroides gingivalis. J Bacteriol. 1990 Mar;172(3):1457–1463. doi: 10.1128/jb.172.3.1457-1463.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano A., Shizukuishi S., Tsunemitsu A., Maekawa K., Tsunasawa S. The primary structure of superoxide dismutase purified from anaerobically maintained Bacteroides gingivalis. FEBS Lett. 1990 Oct 15;272(1-2):217–220. doi: 10.1016/0014-5793(90)80488-5. [DOI] [PubMed] [Google Scholar]
- Aoki H., Shiroza T., Hayakawa M., Sato S., Kuramitsu H. K. Cloning of a Streptococcus mutans glucosyltransferase gene coding for insoluble glucan synthesis. Infect Immun. 1986 Sep;53(3):587–594. doi: 10.1128/iai.53.3.587-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Carlioz A., Ludwig M. L., Stallings W. C., Fee J. A., Steinman H. M., Touati D. Iron superoxide dismutase. Nucleotide sequence of the gene from Escherichia coli K12 and correlations with crystal structures. J Biol Chem. 1988 Jan 25;263(3):1555–1562. [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M. Characterization of the O2-induced manganese-containing superoxide dismutase from Bacteroides fragilis. Arch Biochem Biophys. 1985 Apr;238(1):83–89. doi: 10.1016/0003-9861(85)90143-2. [DOI] [PubMed] [Google Scholar]
- Hassan H. M. Microbial superoxide dismutases. Adv Genet. 1989;26:65–97. doi: 10.1016/s0065-2660(08)60223-0. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Higuchi M. The effect of oxygen on the growth and mannitol fermentation of Streptococcus mutants. J Gen Microbiol. 1984 Jul;130(7):1819–1826. doi: 10.1099/00221287-130-7-1819. [DOI] [PubMed] [Google Scholar]
- Hudson M. C., Curtiss R., 3rd Regulation of expression of Streptococcus mutans genes important to virulence. Infect Immun. 1990 Feb;58(2):464–470. doi: 10.1128/iai.58.2.464-470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Shikita M. Electroblotting of double-stranded DNA for hybridization experiments: DNA transfer is complete within 10 minutes after pulsed-field gel electrophoresis. Anal Biochem. 1990 Feb 1;184(2):207–212. doi: 10.1016/0003-2697(90)90670-5. [DOI] [PubMed] [Google Scholar]
- Keller G. A., Warner T. G., Steimer K. S., Hallewell R. A. Cu,Zn superoxide dismutase is a peroxisomal enzyme in human fibroblasts and hepatoma cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7381–7385. doi: 10.1073/pnas.88.16.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Byers B. R., Olson M. O., Salin M. L., Arceneaux J. E., Tolbert C. A Streptococcus mutans superoxide dismutase that is active with either manganese or iron as a cofactor. J Biol Chem. 1986 Jul 15;261(20):9361–9367. [PubMed] [Google Scholar]
- Martin M. E., Strachan R. C., Aranha H., Evans S. L., Salin M. L., Welch B., Arceneaux J. E., Byers B. R. Oxygen toxicity in Streptococcus mutans: manganese, iron, and superoxide dismutase. J Bacteriol. 1984 Aug;159(2):745–749. doi: 10.1128/jb.159.2.745-749.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Terauchi K., Isobe T., Matsuoka K., Yamakura F. Iron- and manganese-containing superoxide dismutases from Methylomonas J: identity of the protein moiety and amino acid sequence. Biochemistry. 1991 Apr 2;30(13):3210–3216. doi: 10.1021/bi00227a008. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Meier B., Barra D., Bossa F., Calabrese L., Rotilio G. Synthesis of either Fe- or Mn-superoxide dismutase with an apparently identical protein moiety by an anaerobic bacterium dependent on the metal supplied. J Biol Chem. 1982 Dec 10;257(23):13977–13980. [PubMed] [Google Scholar]
- Nakayama K., Irino N., Nakayama H. The recQ gene of Escherichia coli K12: molecular cloning and isolation of insertion mutants. Mol Gen Genet. 1985;200(2):266–271. doi: 10.1007/BF00425434. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Irino N., Nakayama H. recA+ gene-dependent regulation of a uvrD::lacZ fusion in Escherichia coli K12. Mol Gen Genet. 1983;192(3):391–394. doi: 10.1007/BF00392180. [DOI] [PubMed] [Google Scholar]
- Nakayama K. The superoxide dismutase-encoding gene of the obligately anaerobic bacterium Bacteroides gingivalis. Gene. 1990 Nov 30;96(1):149–150. doi: 10.1016/0378-1119(90)90357-w. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989 Feb;3(2):221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Iron- and manganese-containing superoxide dismutases can be distinguished by analysis of their primary structures. FEBS Lett. 1988 Mar 14;229(2):377–382. doi: 10.1016/0014-5793(88)81160-8. [DOI] [PubMed] [Google Scholar]
- Pennington C. D., Gregory E. M. Isolation and reconstitution of iron- and manganese-containing superoxide dismutases from Bacteroides thetaiotaomicron. J Bacteriol. 1986 May;166(2):528–532. doi: 10.1128/jb.166.2.528-532.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellhorn H. E., Hassan H. M. Response of hydroperoxidase and superoxide dismutase deficient mutants of Escherichia coli K-12 to oxidative stress. Can J Microbiol. 1988 Oct;34(10):1171–1176. doi: 10.1139/m88-206. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A. Oxygen metabolism of Streptococcus mutans: uptake of oxygen and release of superoxide and hydrogen peroxide. J Bacteriol. 1983 Jun;154(3):1236–1244. doi: 10.1128/jb.154.3.1236-1244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Van Camp W., Bowler C., Villarroel R., Tsang E. W., Van Montagu M., Inzé D. Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9903–9907. doi: 10.1073/pnas.87.24.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance P. G., Keele B. B., Jr, Rajagopalan K. V. Superoxide dismutase from Streptococcus mutans. Isolation and characterization of two forms of the enzyme. J Biol Chem. 1972 Aug 10;247(15):4782–4786. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lathigra R., Garbe T., Catty D., Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991 Feb;5(2):381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., Pesold-Hurt B., Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]