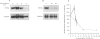Abstract
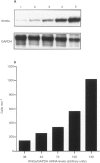
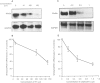
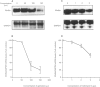
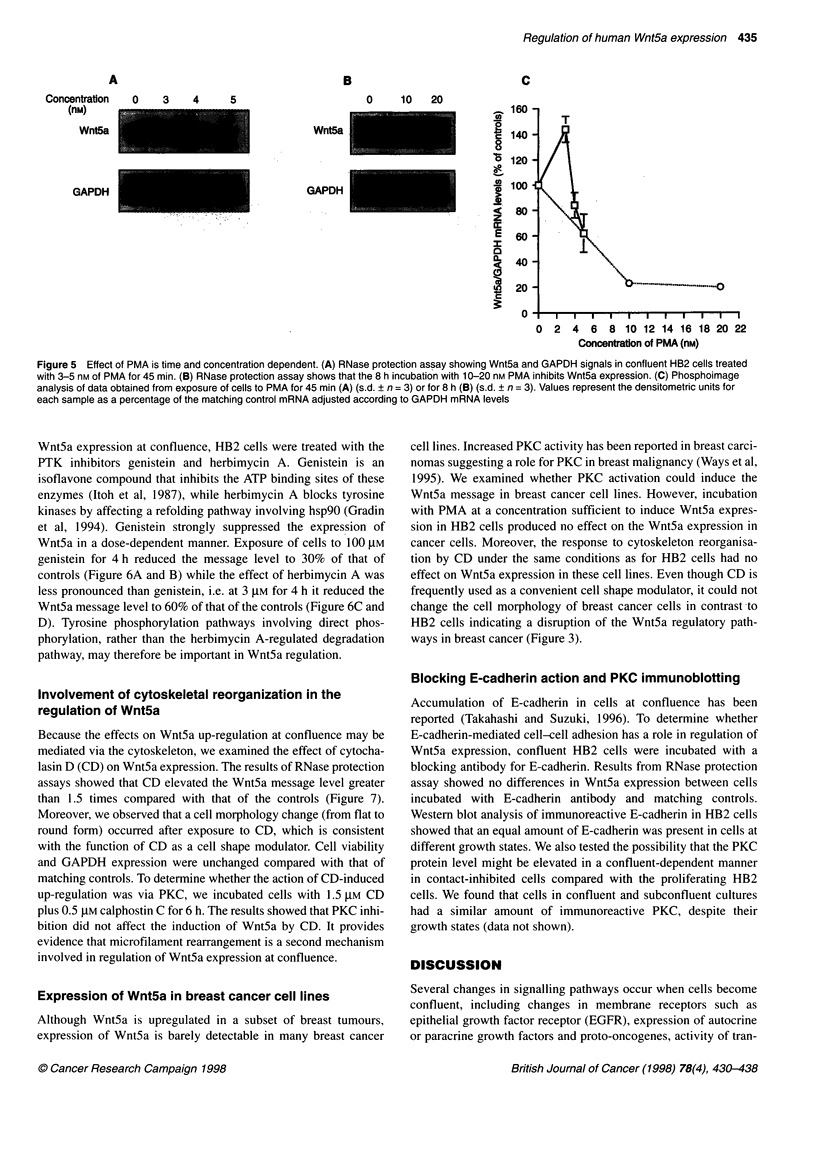
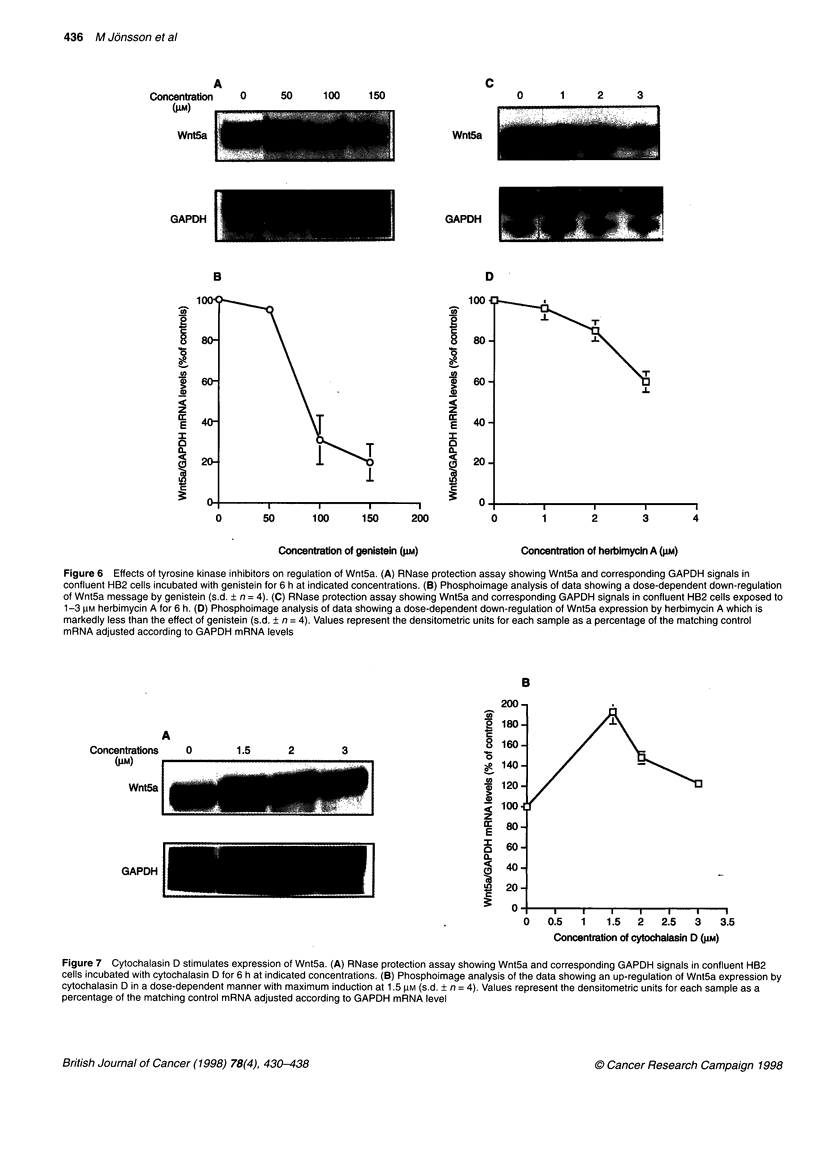
The Wnts can be classified into two classes based on their ability to transform cells. The Wnt5a class can antagonize the effects of transforming Wnts partly through effects on cell migration. To understand the mechanisms of regulation of Wnt5a, we investigated its expression in human normal and breast cancer cell lines. Elevation of Wnt5a in HB2, a normal breast epithelial cell line, was linearly correlated with cell density, but this did not occur in cancer cell lines. We examined intracellular events responsible for the regulation of Wnt5a by cell to cell contacts, using various metabolic agents known to affect signal transduction pathways. Agents that selectively blocked protein kinase C (calphostin C) or protein tyrosine kinases (genistein) reduced the level of Wnt5a expression markedly. Protein kinase C activation by phorbol 12-myristate 13-acetate up-regulated Wnt5a partly through prolongation of Wnt5a mRNA half-life. Cytoskeleton reorganization following cytochalasin D treatment caused an induction of Wnt5a, which was associated with changes in cell morphology. Calphostin C did not block these effects, showing that protein kinase C is acting upstream of cytoskeletal modulation. However, the cancer cell lines treated with cytochalasin D showed no changes in cell morphology or Wnt5a induction, suggesting disruption of this regulatory pathway in cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Bartek J., Bartkova J., Kyprianou N., Lalani E. N., Staskova Z., Shearer M., Chang S., Taylor-Papadimitriou J. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian virus 40 large tumor antigen with a recombinant retrovirus. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3520–3524. doi: 10.1073/pnas.88.9.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost L. M., Hjelmeland L. M. Cell density regulates differential production of bFGF transcripts. Growth Factors. 1993;9(3):195–203. doi: 10.3109/08977199309010832. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay S. M., Tonks N. K. Protein tyrosine phosphatases as adhesion receptors. Curr Opin Cell Biol. 1995 Oct;7(5):650–657. doi: 10.1016/0955-0674(95)80106-5. [DOI] [PubMed] [Google Scholar]
- Clark C. C., Cohen I., Eichstetter I., Cannizzaro L. A., McPherson J. D., Wasmuth J. J., Iozzo R. V. Molecular cloning of the human proto-oncogene Wnt-5A and mapping of the gene (WNT5A) to chromosome 3p14-p21. Genomics. 1993 Nov;18(2):249–260. doi: 10.1006/geno.1993.1463. [DOI] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Cook D., Fry M. J., Hughes K., Sumathipala R., Woodgett J. R., Dale T. C. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J. 1996 Sep 2;15(17):4526–4536. [PMC free article] [PubMed] [Google Scholar]
- Danielson K. G., Pillarisetti J., Cohen I. R., Sholehvar B., Huebner K., Ng L. J., Nicholls J. M., Cheah K. S., Iozzo R. V. Characterization of the complete genomic structure of the human WNT-5A gene, functional analysis of its promoter, chromosomal mapping, and expression in early human embryogenesis. J Biol Chem. 1995 Dec 29;270(52):31225–31234. doi: 10.1074/jbc.270.52.31225. [DOI] [PubMed] [Google Scholar]
- Firth J. D., Ratcliffe P. J. Organ distribution of the three rat endothelin messenger RNAs and the effects of ischemia on renal gene expression. J Clin Invest. 1992 Sep;90(3):1023–1031. doi: 10.1172/JCI115915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis P., Malter J. S. The adenosine-uridine binding factor recognizes the AU-rich elements of cytokine, lymphokine, and oncogene mRNAs. J Biol Chem. 1991 Feb 15;266(5):3172–3177. [PubMed] [Google Scholar]
- Gradin K., Whitelaw M. L., Toftgård R., Poellinger L., Berghard A. A tyrosine kinase-dependent pathway regulates ligand-dependent activation of the dioxin receptor in human keratinocytes. J Biol Chem. 1994 Sep 23;269(38):23800–23807. [PubMed] [Google Scholar]
- He X., Saint-Jeannet J. P., Wang Y., Nathans J., Dawid I., Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997 Mar 14;275(5306):1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P. Identification of the 52 kDa cytoskeletal-like protein of cytochalasin D-stimulated normal rat kidney (NRK/CD) cells as substrate-associated glycoprotein p52 [plasminogen-activator inhibitor type-1 (PAI-1)]. Expression of p52 (PAI-1) in NRK/CD cells is regulated at the level of mRNA abundance. Biochem J. 1992 Jun 1;284(Pt 2):433–439. doi: 10.1042/bj2840433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet E. L., Smith K., Bicknell R., Harris A. L. Regulation of Wnt5a mRNA expression in human mammary epithelial cells by cell shape, confluence, and hepatocyte growth factor. J Biol Chem. 1995 May 26;270(21):12851–12856. doi: 10.1074/jbc.270.21.12851. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Eichstetter I., Danielson K. G. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995 Aug 15;55(16):3495–3499. [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Lejeune S., Huguet E. L., Hamby A., Poulsom R., Harris A. L. Wnt5a cloning, expression, and up-regulation in human primary breast cancers. Clin Cancer Res. 1995 Feb;1(2):215–222. [PubMed] [Google Scholar]
- Li W., Ohlmeyer J. T., Lane M. E., Kalderon D. Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell. 1995 Feb 24;80(4):553–562. doi: 10.1016/0092-8674(95)90509-x. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Campbell R. M., Christian J. L., McGrew L. L., Shih J., Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993 Sep;119(1):97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Wnt genes. Cell. 1992 Jun 26;69(7):1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Ohh M., Smith C. A., Carpenito C., Takei F. Regulation of intercellular adhesion molecule-1 gene expression involves multiple mRNA stabilization mechanisms: effects of interferon-gamma and phorbol myristate acetate. Blood. 1994 Oct 15;84(8):2632–2639. [PubMed] [Google Scholar]
- Shimoyama Y., Hirohashi S., Hirano S., Noguchi M., Shimosato Y., Takeichi M., Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989 Apr 15;49(8):2128–2133. [PubMed] [Google Scholar]
- Soprano K. J. WI-38 cell long-term quiescence model system: a valuable tool to study molecular events that regulate growth. J Cell Biochem. 1994 Apr;54(4):405–414. doi: 10.1002/jcb.240540407. [DOI] [PubMed] [Google Scholar]
- Stephens J. M., Carter B. Z., Pekala P. H., Malter J. S. Tumor necrosis factor alpha-induced glucose transporter (GLUT-1) mRNA stabilization in 3T3-L1 preadipocytes. Regulation by the adenosine-uridine binding factor. J Biol Chem. 1992 Apr 25;267(12):8336–8341. [PubMed] [Google Scholar]
- Takahashi K., Suzuki K. Density-dependent inhibition of growth involves prevention of EGF receptor activation by E-cadherin-mediated cell-cell adhesion. Exp Cell Res. 1996 Jul 10;226(1):214–222. doi: 10.1006/excr.1996.0221. [DOI] [PubMed] [Google Scholar]
- Torres M. A., Yang-Snyder J. A., Purcell S. M., DeMarais A. A., McGrew L. L., Moon R. T. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996 Jun;133(5):1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vider B. Z., Zimber A., Chastre E., Prevot S., Gespach C., Estlein D., Wolloch Y., Tronick S. R., Gazit A., Yaniv A. Evidence for the involvement of the Wnt 2 gene in human colorectal cancer. Oncogene. 1996 Jan 4;12(1):153–158. [PubMed] [Google Scholar]
- Ways D. K., Kukoly C. A., deVente J., Hooker J. L., Bryant W. O., Posekany K. J., Fletcher D. J., Cook P. P., Parker P. J. MCF-7 breast cancer cells transfected with protein kinase C-alpha exhibit altered expression of other protein kinase C isoforms and display a more aggressive neoplastic phenotype. J Clin Invest. 1995 Apr;95(4):1906–1915. doi: 10.1172/JCI117872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie B., Bucana C. D., Fidler I. J. Density-dependent induction of 92-kd type IV collagenase activity in cultures of A431 human epidermoid carcinoma cells. Am J Pathol. 1994 May;144(5):1058–1067. [PMC free article] [PubMed] [Google Scholar]
- Young S., Rothbard J., Parker P. J. A monoclonal antibody recognising the site of limited proteolysis of protein kinase C. Inhibition of down-regulation in vivo. Eur J Biochem. 1988 Apr 5;173(1):247–252. doi: 10.1111/j.1432-1033.1988.tb13991.x. [DOI] [PubMed] [Google Scholar]
- Zambetti G., Ramsey-Ewing A., Bortell R., Stein G., Stein J. Disruption of the cytoskeleton with cytochalasin D induces c-fos gene expression. Exp Cell Res. 1991 Jan;192(1):93–101. doi: 10.1016/0014-4827(91)90162-n. [DOI] [PubMed] [Google Scholar]